
Leica VT1200 S Fully automated vibrating blade microtome
The fully automated Leica VT1200 S vibrating blade microtome is designed for sectioning fresh specimens in neuroscience. Automatic feeding, specimen retraction and the use of a cutting window help to minimize sectioning time. The instrument is recommended for multi-user laboratories where both the semi-automated and fully automated functions are needed.
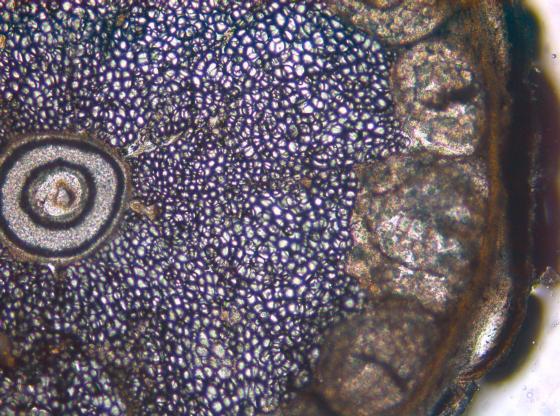
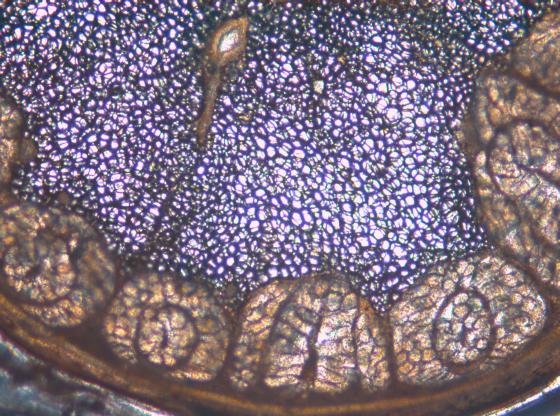
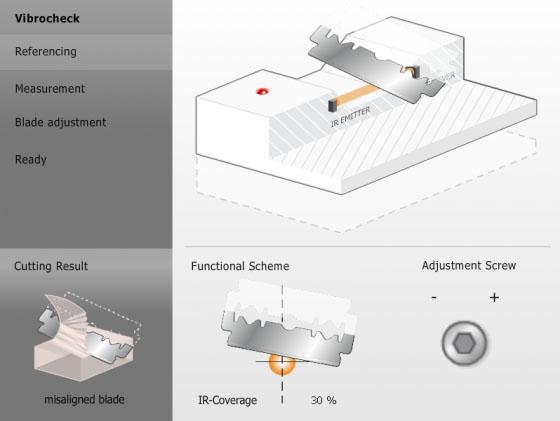
To achieve sections that retain viable cells on the section surface, the vertical deflection of the blade can be measured with the Leica Vibrocheck measurement device and minimized with the innovative blade holder.
The instrument was designed in collaboration with Prof. Dr. Peter Jonas (previously at the Physiology department of the University of Freiburg, Germany, now at Institute of Science and Technology, Klosterneuburg, Austria) and his former group.
Technical Specifications
GENERAL
| Sectioning frequency: | 85 Hz (± 10 %) |
| Amplitude: | adjustable from 0 - 3 mm, in 0.05 mm increments |
| Sectioning speed: | adjustable from 0.01 - 1.5 mm/s (± 10 %) |
| Return speed: | 2.5 mm/s (± 10 %) |
| Total vertical specimen stroke: | 20 mm (motorized) |
| Cutting range: | 45 mm (adjustable) |
| Weight (Basic Instrument): | 56 kg |
| Dimensions L x W x H (Basic Instrument): | 600 mm x 250 mm x 230 mm |
Product Features
Minimal specimen damage
Minimal vertical deflection minimizes specimen damage during sectioning and results in more viable cells on the section surface.
Accommodates sectioning preferences
Semi and fully automated cutting modes accommodate many user’s sectioning preferences.
Double edged razor injector
Blade holder for double edged razor, injector or sapphire blades supports safer blade handling. A second blade holder helps to avoid contamination of specimens.
Built-in software
Built-in software remembers individual operational parameter settings for up to 8 users.
Magnetic specimen holders
Magnetic specimen holders support easy specimen handling and reproducible orientation.
Downloads
Brochure
Certificate
IFU
Case Studies & White Papers
Configurations
Basic instrument (14048142066)
- Leica VT1200 S – basic instrument without blade holder, ice tray, buffer tray - metal, or specimen disc
- Fully automated Vibrating Blade Microtome for sectioning unfixed specimens from Neuropathology and Neurophysiology (Patch clamping).
- Vertical specimen feed: max. 20 mm
Configuration 1 (1491200S001)
- Leica VT1200 S - Configuration with blade holder, ice tray, buffer tray - metal, and specimen disc
- Fully automated Vibrating Blade Microtome for sectioning unfixed specimens from Neuropathology and Neurophysiology (Patch clamping).
- Robust and stabile basic instrument with blade holder for safe insertion of the whole razor blade and adjustment screw to minimize the vertical deflection of the blade below 1 µm.
- Vertical specimen feed: max. 20 mm.
Leica VT1200 and VT1200 S Parts/Accessories