
Special Stains – Which One, How and Why? Part II: Connective Tissue

Definition of "Special Stain"
"Special stain" is a term used to refer to many alternative staining techniques that are used when the traditional H&E does not provide all the information the pathologist or researcher needs from a tissue slide. "Special stains" are processes that generally employ a dye or chemical that has an affinity for the particular tissue component that is to be demonstrated. They allow the presence/or absence of certain cell types, structures and/or microorganisms to be viewed microscopically. "Special stains" are not the same as and existed long before immunohistochemical (IHC) and/or molecular techniques (probes).
Connective Tissue - Introduction
Connective tissue supports the body by providing a matrix that connects and binds the cells and organs. There are three types of connective tissue in the body.
Collagen is a strong protein and is a main component of ligaments and tendon. It is also responsible for skin elasticity and is the most visible type which can be visualized with an H&E.
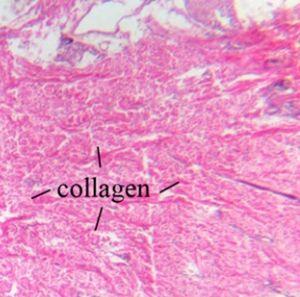
Elastic fibers are located in the skin and walls of blood vessels. They are composed of elastin - a protein that is flexible and allows many tissues in the body to resume their shape after stretching or contracting and are not visualized by an H&E.
Reticular fibers are composed of collagen and form a delicate framework around nerve fibers, fat cells, lymph nodes, and smooth and skeletal muscle fibers. They are also not visualized by an H&E.
The Most Commonly Used Stains to Demonstrate Connective Tissue
Trichrome - Masson
Three dyes are used to selectively stain including muscle (red), collagen fibers (blue) erythrocytes (red) and nuclei (black).
The dyes are used to stain liver biopsies to determine the degree of fibrosis as in cirrhosis as well as kidney biopsies to accentuate the basement membrane and demonstrate immune deposits. The dyes are also used to differentiate between smooth muscle and collagen in tumors and verify increase of collagen.
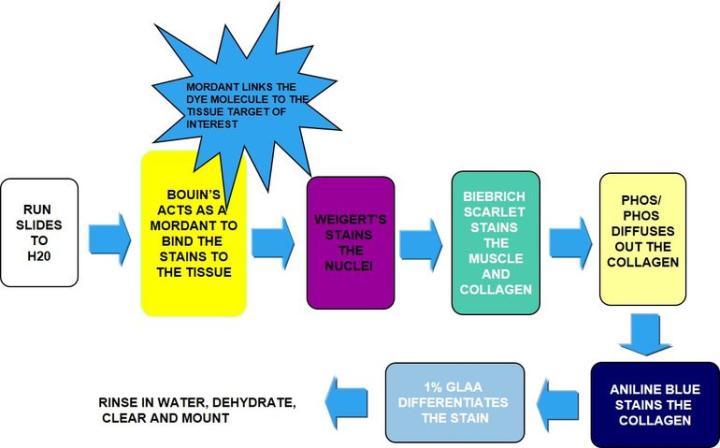
The Process
The Results

The Protocol – Incubation times may vary per manufacturer guidelines
|
1. |
Bring slides to water |
|
|
2. |
Incubate in Bouin's – acts as a mordant to bind the dye to the tissue. |
|
|
Usually overnight |
|
|
10-15 mins |
|
|
3. |
Rinse well in running water to remove all traces of Bouin's |
|
|
4. |
Weigert's hematoxylin |
5-10 mins |
|
5. |
Rinse in running water |
5 mins |
|
6. |
Biebrich scarlet |
15 mins |
|
7. |
Rinse in running water |
|
|
8. |
Phosphotungstic-phosphomolybdic acid |
15 mins |
|
9. |
Straight into aniline blue (no rinse) |
5 mins |
|
10. |
Straight into 1% glacial acetic acid |
1-3 mins |
|
11. |
Rinse in running water |
|
|
12. |
Dehydrate, clear and mount |
Troubleshooting the Trichrome:
It is essential to use good Bouin's with proper incubation times for the appropriate temperature used. Complete differentiation with Phosphotungstic-phosphomolybdic acid of collagen to the point that all red is removed. If muscle red is pale, check viability of Bouin's and/or Biebrich scarlet.If collagen is too pale, decrease incubation time in 1% Glacial Acetic Acid (GLAA).
One Step Trichrome - Gomori's
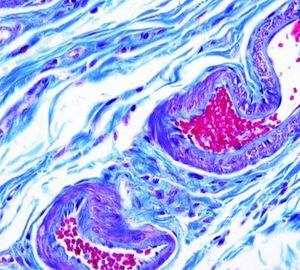
One solution stains all: muscle, collagen fibers, fibrin and erythrocytes. There are two formulations to stain collagen: blue and green.
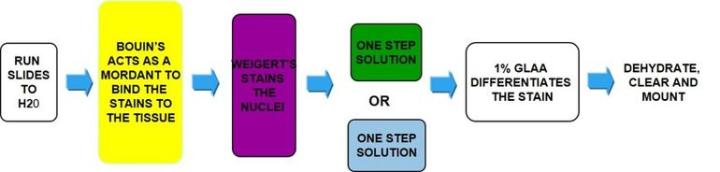
The Process
The Results
The Protocol
Incubation times may vary per manufacturer guidelines.
|
1. |
Bring slides to water |
|
|
2. |
Incubate in Bouin's – acts as mordant to bind the dye to the tissue |
|
|
usually overnight |
|
|
10-15 mins |
|
|
3. |
Rinse well in running water to remove all traces of Bouin's |
|
|
4. |
Weigert's hematoxylin |
5-10 mins |
|
5. |
Rinse in running water |
5 mins |
|
6. |
One step blue/green solution |
15 mins |
|
7. |
Straight to 1% glacial acetic acid |
1 min |
|
8. |
Rinse in running water |
|
|
9. |
Dehydrate, clear and mount |
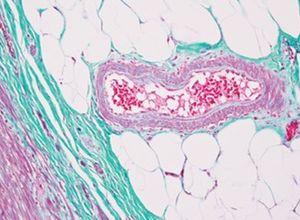
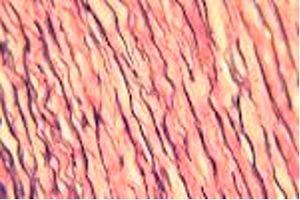
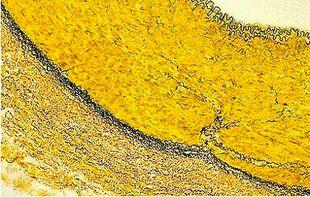
Verhoeff-van Gieson's Elastic Stain
Verhoeff-van Gieson's Elastic Stain is used to identify the atrophy of elastic tissue as in emphysema, evidence of vascular diseases (arteriosclerosis), and the invasion of tumors into vessels.
The Process
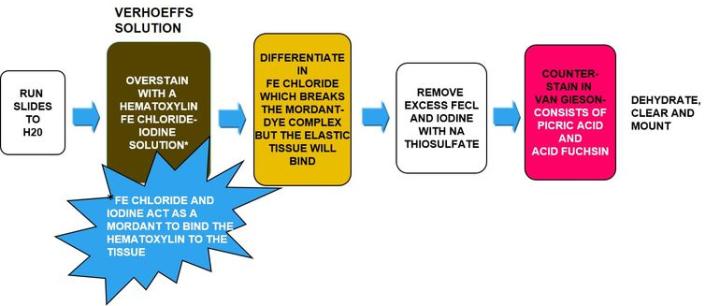
The Results
The Protocol
Incubation times may vary per manufacturer guidelines.
|
1. |
Bring slides to water |
|
|
2. |
Verhoeff elastic stain-working solution (per manufacturer) |
15 min |
|
3. |
Rinse well with running water |
|
|
4. |
Differentiate in 2% ferric chloride |
1-2 mins |
|
5. |
Rinse in running water |
5-10 mins |
|
6. |
5% sodium thiosulfate |
1 min |
|
7. |
Rinse in running water |
|
|
8. |
Counterstain in van Gieson's solution |
30 sec -1min |
|
9. |
Skip 95% and quickly dehydrate in 100 x 2 |
|
|
10. |
Clear and mount |
Troubleshooting the Elastic Stain:
Verhoeff’s solution needs to be fresh each use due to rapid oxidation. Do not over differentiate in the ferric chloride as the dye molecules love this stuff equally as well as they do the tissue molecules they are bound to and will disconnect from the tissue easily. If this happens you will have to start all over. Incubate for a few seconds then rinse quickly and view microscopically looking for the elastic tissue fibers. When they are black and easily differentiated from collagen and other tissue types, stop the process.
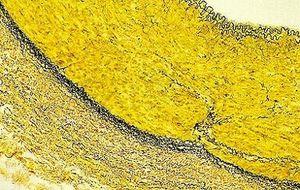
Likewise, do not incubate too long in the van Gieson as the picric acid in solution will continue to differentiate out the bound hematoxylin.
Gomori's Reticulin Fiber
Reticulin fibers support the body and are common in the liver, spleen and kidneys. Characteristic reticulin patterns can help diagnose cirrhosis of the liver, early fibrosis in bone marrow, tumors including: hemangiosarcomas - tumor of cells that line blood vessels, fibroblastic tumors, and rhabdomyosarcomas - tumor of muscles that are attached to bone. They can also help diagnose epithelial versus non-epithelial tumors.
Reticulin stains use silver and rely on the argyrophilic properties of the fibers. Argyrophilic cells can adsorb silver but cannot reduce it. "Adsorb" is a surface-based process, whereas "absorb" means there is permeation through the tissue. Argyrophilic cells need a reducer/developer to convert the invisible silver salts to a visible metallic silver.
The Process
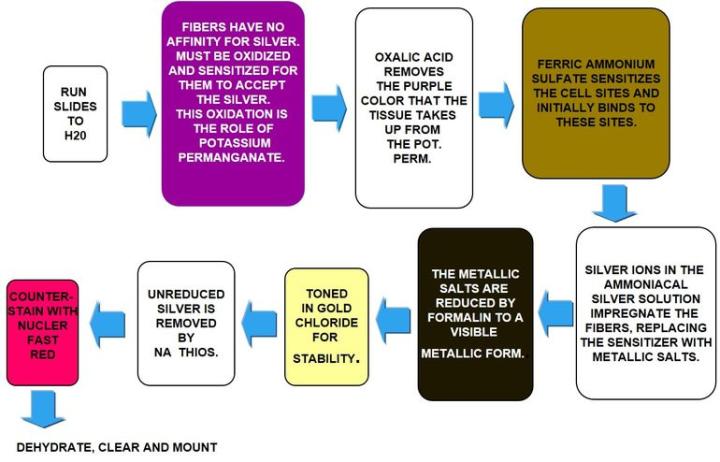
The Results
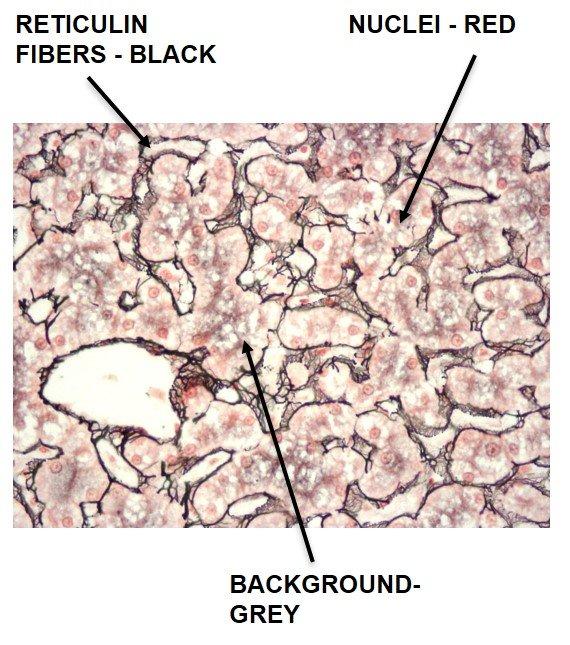
The Protocol
|
1. |
Bring slides to water |
|
|
2. |
Oxidize in potassium permanganate |
10 min |
|
3. |
Rinse well in running water |
3 mins |
|
4. |
Oxalic acid |
1 mins |
|
5. |
Rinse well in running water |
2 mins |
|
6. |
Sensitize in ferric ammonium sulfate |
15 mins |
|
7. |
Rinse in distilled water |
2 mins |
|
8. |
Impregnate in ammoniacal silver |
2 mins |
|
9. |
Rinse in distilled water |
1 min |
|
10. |
Develop in 20% unbuffered formalin |
1 min |
|
11. |
Rinse well in running water |
2 mins |
|
12. |
Tone in 0.1% gold chloride |
3-5 mins |
|
13. |
Rinse in water |
1 min |
|
14. |
5% sodium thiosulfate |
1 min |
|
15. |
Rinse in water |
1 min |
|
16. |
Counterstain in nuclear fast red |
3-5 mins |
|
17. |
Rinse briefly in water |
15 sec |
|
18. |
Dehydrate, clear and mount |
Troubleshooting the Gomori’s reticulin:
Silver solution should be made fresh with distilled water and clean glassware (acid clean is not necessary). Take care to make the silver. Using old, ammonia depleted ammonium hydroxide can cause the solution to fail. Do not use excessive ammonium hydroxide to clear the silver/sodium hydroxide solution. Excess ammonium hydroxide will cause weak staining.
How to make ammoniacal silver:
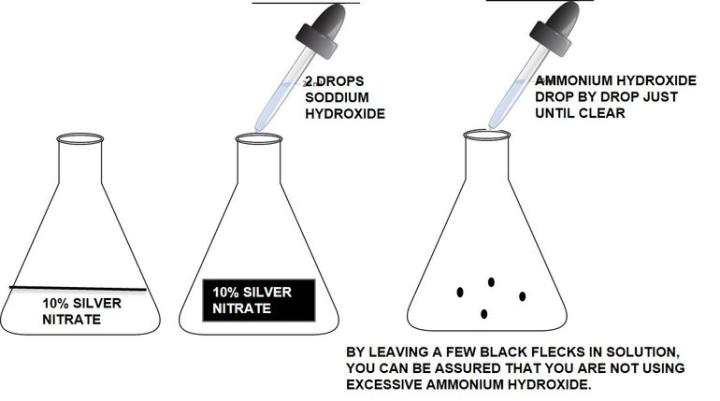
How to Demonstrate Collagen, Elastic Fibers, Reticular Fibers, Nuclei and Muscle with One Procedure
Movat Pentachrome
Movat Pentachrome is a super connective tissue stain. It is used to study heart tissue, blood vessels and vascular and lung diseases. Due to the excellent differentiation of collagen, elastic, fibrin and muscle fibers, the Movat can reveal subtle changes that the routine and normal special stains do not.
The Process
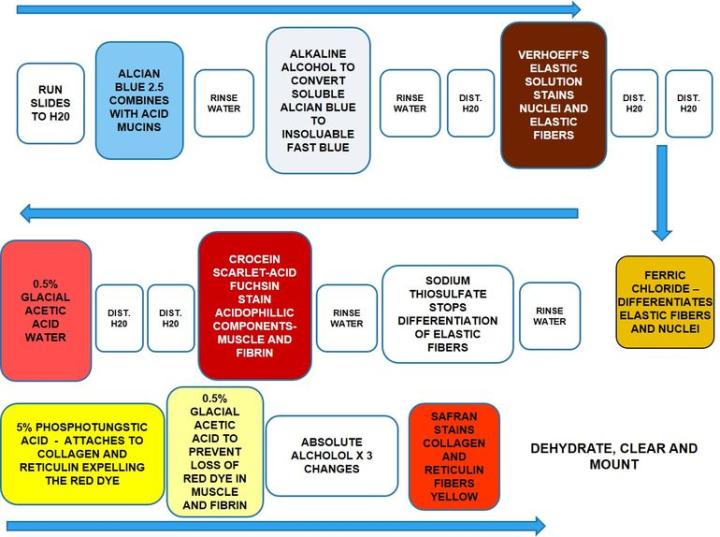
The Results

The Protocol
Incubation times and reagent sequence may vary per manufacturer, but results are similar.
|
1. |
Bring slides to water |
|
|
2. |
Alcian blue |
20 mins |
|
3. |
Rinse well in running water |
5 mins |
|
4. |
Alkaline alcohol |
60 mins |
|
5. |
Rinse well in running water |
10 mins |
|
6. |
Rinse in distilled water |
|
|
7. |
Verhoeff's elastic solution |
12-15 min |
|
8. |
Distilled water x 2 |
|
|
9. |
Ferric chloride |
2 to 3 dips |
|
10. |
Rinse in distilled water |
|
|
11. |
Sodium thiosulfate |
1 min |
|
12. |
Rinse well in running water |
5 mins |
|
13. |
Croceine scarlet – acid fuchsin |
90 sec - 3 min |
|
14. |
Distilled water x 2 |
|
|
15. |
.5% acetic acid water |
|
|
16. |
5% aqueous phosphotungstic acid x 2 |
5 min each |
|
17. |
.5% acetic acid water |
|
|
18. |
Absolute alcohol x 3 |
Quick dips |
|
19. |
Safran |
15 min |
|
20. |
Absolute alcohol x 2 |
|
|
21. |
Clear and mount |
|
Troubleshooting the Movat Pentachrome
Alcian blue should be used at PH 2.5. Complete removal of alkaline alcohol is essential for good staining. Verhoeff’s solution should be made fresh before each use as it oxidizes fast. Be careful not to over differentiate elastic fibers in ferric chloride. Dip once or twice then rinse in distilled water and check microscopically. Repeat as necessary. Do not use graded alcohols to dehydrate but start in absolute alcohol. Do not allow sections to dry out during the staining procedure.
About the presenter

Carolyn Doan received her ASCP registration in 1969 after completing classes at Georgia State University and St Joseph school of Histotechnology in Atlanta, Georgia. For 40 years she shared her passion for histology in research (at Yerkes Primate Research Center), in the clinical world (managing the Pathology Department at Florida Hospital in Orlando and serving on the board of the Florida State Licensure Task Force and as president of the Florida State Society for Histotechnology), and in industry (as a Sales executive, Marketing Manager and North American staining sales specialist). Since her retirement in 2013, she founded Creative Histology Consulting.
References
Bibliography
Rolls, Geoffrey. “101 Steps to Better Histology – A Practical Guide to Good Histology Practice”. Knowledge Pathway/Training Resources.
Shaikh, Imran. “Special Stains in Histopathology” Kem Hospital, 2012.
Sheehan, D. “Theory and Practice of Histotechnology.” Connective Tissue. 2nd edition, 10: 180-200.
Culling, C., et.al. Cellular Pathology Technique, edition 4. Butterworths, London, England 1985.
Wikipedia – Ammoniacal Silver, Nov 2013.
Russel, H.K. A Modification of the Movat Pentachrome Stain. Arch Pathol, 94: 187, 1972.
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.