Aperio GT 450 Workflow Research Study


Webinar Transcription
Welcome everyone to the Aperio GT 450 workflow research study. My name is Julie Broccardo and I am the director of anatomic pathology operations for NeoGenomics Laboratories. I am currently responsible for managing multi-site operations for Neo, which include our Fort Myers, Houston, Carlsbad and Aliso Viejo AP laboratories. I'm very passionate about working with my teams across all the sites and working on initiatives to help build and develop a more efficient lab for Neo.
Dr. Chizhevsky and I are very excited to share with you all today the results from the GT 450 workflow study that Neo was able to participate in. This was using the Aperio GT 450, which is research use only, and our study was for research use only.
This study for us started back in 2014 and NeoGenomics at the time was looking for a partnership on how to work with Leica on our image analysis solutions. In 2014, we took the day and went down to the Vista office with Lance and his team. We got to sit with them and discuss issues, things of collaboration, stuff we wanted to see for future for Neo for the needs for scalability. We had a good day, and we left there being able to give our customer feedback to them. Things that we wanted to discuss was how to make the imaging for my operations much easier, faster and so these are the voice of the customer in which we were able to provide to Leica.
If we fast forward to 2017, Neo was working with myself and my team as volumes were heavily increasing and the senior leadership team had come to me, looking for an answer on what we can do to scale up our digital imaging operations. We needed something with scalability, with quality. This is where I started looking at vendors and about that time I got a phone call from Lance telling me, “Hey, Julie, we've got this new scanner and I'd like you to come down to the Vista office to come see it.”
I was very intrigued and he's telling me he has this new scanner that can do 81 slides per hour. It can scan everything at 40X. It scans in 32 seconds. I was intrigued. I immediately went down to the Vista office and met with Lance and his team and started, getting into the scanner and reviewing essentially what Lance was telling me over the phone that could do.
Benefits for Histotechnicians
This scanner, as we watched it in the like office, it was great and it was amazing. It was almost too good to be true. We got to see it scan at 32 seconds. There's the no-touch operations, which is nice. You can do continuous loading where you don't have to stop the scanner and wait to load another slide. It just is continual loading. You could touch the scanner, tell it to stop scanning and prioritize something else. You could scan in batches, in singles. The capacity was increased to 450 slides.
A big one that I was really impressed with is that the scanner was able to identify its own image quality and rescan itself if it was needed. And essentially, I was hooked. It was love at first sight, and I was very, very excited to the point where I was very persistent with Lance to see if I could get one of these scanners to take home with me that day. He graciously declined. But then I was persistent as I wanted to work with this GT 450, but I wanted to work with it in my environment at Neo and see how it really compared with our settings our network, because everybody has a different setup and solution. I wanted to see how this new instrument would be able to work and behave in our environment.
Samples
Leica at the time agreed to participate and let Neo participate in a workflow study including the GT 450 with our AT2 scanners in Neo. To go over our study design, we selected 155 slides to be a representative sampling for this study. We picked out of these 155 slides, representative sections or things that we saw in our daily operations. This included H&Es, breast panels, colon markers and our HPV ISH cases. We selected 35 different tissues and stained them with the H&E and scanned them. We had 36 breast IHC markers and their associated H&Es. We added 26 colon markers with their associated H&Es as well as 33 CISH HPV slides with their associated H&E's to this study.
In addition to the slides, we made sure that we had more than one person participating. We had three techs of all different knowledgebase levels of imaging partake to make sure that we had a good metric that we're getting. Not somebody who only knows imaging and who can push through cases quicker than somebody who just is barely learning. We wanted to make sure we had all different skill sets partake in this study.
You can see out of the 35 H&Es, we took different selections of soft tissue and bone. These were representative tissues that come through Neo and we stained with H&E and we're able to scan them just reviewing the H&E image. You can see the 36 breast markers that we ran comprised of essentially 10 cases. In those cases, is essentially these breast panels. And so the breast panels included are HER2, ER, and PR, Ki67, and/or p53. Each case is essentially set up differently with a different configuration. Again, we were trying to simulate what we see in our daily operations within Neo.
For the colon markers, we had eight cases of the MMR panel, and again we made the panels variants of combinations as this is what we see in our normal operations. And so this MMR was including the MLH-1 MSH-2, MSH-6, and PMS2 with their associated H&Es and the same was with the breast panels. Previously we ran their associated H&Es within each case. Lastly, we added our FISH HPV markers to the study so we ran 7 cases comprising of our RNAScope HPB ISH model with their associated H&Es.
Equipment
The equipment used to perform this workflow study was our AT2 scanners that we currently have and obviously an Aperio GT 450 with its SAM server. Software that was used to perform the scanning was the Aperio eSlideManager and to review the images we use the Aperio ImageScope to review the overall image quality.
The equipment setup and design of this workflow is we made sure we pulled an AT2 out of our current workflow production and set it aside to be part of this study along with the GT 450. We tried to simulate our AT2s as much as possible to simulate the workflow of the GT 450. Being able to scan, go to the SAM server and into the pathologist office.
Equipment Setup
The same was with the AT2 as it was, scanning, going through our typical network in which the SAM server did as well and going to the pathologist suite for them to be able to review, the images on the back end.
AT2 Workflow Steps
To do this study, we had to be able to identify the workflow steps that we needed to measure between the AT2 and the GT450. So here you can see for the AT2 we defined ten workflow steps that are specific to Neo and our current workflow that we currently have daily. With that, we had to also define the categories of those workflows so we knew what metrics we were going to be measuring. Hands on time versus QC time, scan time, etcetera.
As you can see, we have ten workflow steps for the AT2. This workflow step starts from a procedure after the slides come off the instruments and we do an initial review of the staining quality upstream. Once that team approves them, they will then push them downstream to the imaging department and we start step one.
The imaging team will receive the slides in slats. They will then start the time at the time of opening up those slats and cleaning the slides. They make sure the slides are clean. Once they're clean, they put them into the AT2 racks. They will then load those racks into the Aperio AT2 scanners and then select the pre snap. After the pre snap is done, they will then go in and review the image and manually adjust the scan area, the green box over the tissue. Then manually adjust and add or remove focus points.
Once that is complete, the tech will then start the scanning which we scan on the AT2 at 20x. While that is scanning, they wait. When the scanning is done, they come back and then they will review each slide image and they look for five image points to QC before they move on to the next image. What they're QC’ing is overall image quality. Making sure nothing is blurry, that a focus point didn't land on something in which we're going to have to repeat the scan.
Once they confirm the image quality is good, they will then remove the racks from the AT2 and then they will take the slides out of the racks and put them back into their corresponding flats.
Aperio GT 450 Workflow Steps
Here you can automatically or right away see that we have six workflow steps with the GT450 in comparison to the AT2 in which we had ten workflow steps. Right off the bat I was excited and wow, that I'm going to have less steps involved in this scanner. That means less tech time applied, hopefully less tech time. That's the assumption being made. We had six steps that we had to go through same as the AT2 and they were essentially handed down the same. So the flats were handed down to the imaging team, they had to clean the slides. Once they were clean, they would insert the slides into the Aperio GT 450 racks and then they were able to load the GT 450 and which was nice about the GT 450 is it was continuous loading.
We didn't have to stop or pause like we do with the AT2 to add more. We were able just to continually load the scanner. As the scanner has already started the scanning process, so as we're loading the instruments started scanning once the scans became available, the tech would then review in the Tissue Finder the overall macro image for each slide.
Once they were able to confirm that image quality was good, they would then move it on to removing them from the rack. They were not having to do the five workflow steps of the image quality. Essentially the instrument was doing all the image quality for us and if there was an image that needed us to be reviewed, it would highlight itself and rescan.
Then the tech would then go and remove the racks from the GT 450 and place those slides back into the flats. And again, that's when we stopped the time. You can see just before even starting this study I was really excited to see that with this new scanner there was going to be less steps in the workflow that we would have to manage.
Study End Points
The categories in which we wanted to measure are these five metrics. So we wanted to capture the QC tech time, the tech hands on time, the total turn around time, the slide throughput and the image quality between the AT2 and the GT 450. We wanted to really look at these five things as they're important to our operations and to our pathologists. The goal is to make sure we can see efficiencies, reductions in certain areas, but all the while maintaining image quality.
QC tech time was the time measured in which our operator spent reviewing or performing some quality function. This was measured in hours and minutes. This is the time on the AT2s in which we had to do the pre snap, select the scan area, or at the back end where we were having to review the images and select five different regions to QC. Whereas in the GT 450 we just have to overall look at the image provided by the scanner and make sure it meets our quality needs.
The hands-on time was the actual aggregate measurement of time that the tech physically was handling material. So cleaning slides, loading the scanners, selecting the regions to be scanned, removing the slides, and replacing them in the flats. As I showed you previously we had a decrease in these workflow steps with the GT450 in comparison to the AT2. To reiterate, the hands-on time does not include the step in which the scanner is performing its scanning functions. The turnaround time is the total time that we calculated in hours, minutes, and seconds from the very beginning to the very end. This included the hands-on, the QC, and the scanning time.
Another metric we wanted to look at was the slide throughput. We wanted to see the difference between the two scanners and see how many slides could be pushed through the instruments per hour. Lastly was the image quality. We did this based reviewing with our medical team and we had different pathologists participate and have them look at the overall quality scores between the two different scanners.
Results: QC Tech Time
Going into the results of the QC tech time was pretty significant. We weren't expecting this much of a reduction. From our study, we were able to see a 94% reduction in the tech QC time applied to this study. If we look at these differences on an average, it took with an AT2 an hour and 25 minutes to QC the 155 slides, whereas the GT 450 took us five minutes.
With this reduction you will see in the AT2 we had to spend time QC’ing the slides upstream: doing the pre snap, selecting the scan area, adding focus points. Then on the back end, having to review the images because we don't have an instrument that's telling us that there is an issue or that there are acceptable to move forward. When you go to the GT 450, one of the workflow steps that was eliminated was performing those beginning pre snaps, adding the focus points in the scan area because the GT450 has its own tissue finder and does this automatically. We're eliminating those three steps that are done in the AT2. GT 450 does it on its own. Then on the back end, GT450 automatically does its own image quality. We're not having to go in and look at every five areas of an image. The scanner is going to tell us if there is a hiccup, if it had to rescan itself and it gives us a color coding to say, “Hey, you really need to look further into the image.” Whereas if it gives us the green light, we're doing an overall image review and it's very quick and that's why we saw a major reduction in this QC tech time.
Results: Hands on Time
For the results for our hands on time, we saw a 70% reduction with the GT 450 in comparison to the AT2. With AT2, about 29% of our time within this workflow is applied to hands on tech time, whereas with the GT 450 it was decreased to 14%. To understand why we have this decrease is with the AT2 scanner, there was the categories of the pre snaps, the focus areas, the assigning the slide area. These were QC and hands on tech time that we measured because the techs are having to spend time to physically either select the pre snap, select the scan area, adjust the focus points while they are performing that QC function and so when you get to the GT450 this goes away. Those steps are eliminated as the instrument has its own tissue finder and performs these functions for us. These are hands on time reductions that we automatically see with this instrument.
Results: Throughput
The next metric that I want to review is our slide throughput. Between AT2 and the GT 450, we saw 64% slide throughput improvement with the GT 450. As you can see on the graph with our AT2 we scanned at 20x and we were able on average to push 25 slides through the AT2 at 20x in an hour as compared to the GT 450 we were able to scan and push through the scanner approximately 41 slides an hour. This is was 40x compared to the AT2 20x and if you guys have scanners out there, whether it's Aperio, another user, you have to see like we have seen 20X and 40X scan times can greatly vary as you increase the magnification.
It was great to see this study and how we set it up in our real environment, as the GT450 is an automatic default to 40x. To see that it was able to increase that slide throughput at a higher magnification per hour was a real impressive result that I was happy to see.
Results: TAT
The last metric that I'm going to review with you guys was our overall turnaround time. We had a 39% reduction with the GT 450 in comparison to the AT2. The turnaround time was the beginning step from getting the slide cleaning all the way through hands-on time, QC time, and scanning time all the way until we took the glass off the scanners and put them back in the flats. On average, it took us a little over six hours to be able to scan these 155 slides on the AT2. With the GT 450, we were able to decrease that into a little under four hours. This reduction in turnaround time is very helpful for me to help manage the department, to look at investing in this efficiency game because I'm going to be able to get cases pushed through these scanners into our medical team much quicker. This was another impressive result we were able to get from this workflow study.
We had different operators. With turnaround time, we didn't have just one, efficient, fast-running person who understands imaging who can knock this out. We made sure that we had different levels participate so we could get a good average of pushing these cases through both scanners.
Using Integrated Racks Could Further Reduce TAT
If I can refer to our metric regarding the hands-on tech time, we saw a reduction of 70% from the AT2 to the GT 450. With the GT 450, we were still having to apply 14% of our tech time to the workflow steps. This was part of our workflow, still currently is, but there is room for improvement, meaning there's additional automation out there that is upstream of the scanners that could help if implemented into workflow, possibly even reduce our workflow steps and hands on time even further.
Summary
In summary, we saw a significant increase in our overall slide throughput and a significant decrease in our QC time, our hands-on time, and our overall turnaround time. All the while sustaining and maintaining our overall image quality. As an operational leader, I'm always looking to identify efficiency gains within our lab to help improve our overall productivity. As our volumes grow, I can't always add headcount to our capacity constraints and continue to look for efficiency gains wherever available.
This GT 450 has allowed us to relocate resources that we currently have in our imaging department and move them upstream. Instead of adding headcount on the back end, we have moved them upstream within the microtomy and staining regions in which we need help with those increasing volumes. While the GT 450 is now able to manage the back-end capacities without needing as much tech time available to operate these instruments. This is a huge advantage of having this GT 450 in our workflow.
OK, so at this time I'm going to hand it over to Doctor C to discuss with you all the details and results around our image quality metrics that we captured in this workflow study.
Hello and thank you for joining us for digital pathology virtual summit. I hope everybody is coping well in these trying times. My name is Vladislav Chizhevsky. I'm a director of hematopathology and a senior pathologist at NeoGenomics Laboratories. I have been involved in digital pathology for many years and am very excited to speak with you about the image quality that the GT 450 can deliver.
Image Quality Considerations
In the previous slides, Julie showed you the technical advantages of the Aperio GT 450. However, the Aperio GT 450 takes the digital technology even further with excellent images and crisp detail. In the next few slides, we will show you the detail and the precision of this instrument.
Benefits for Pathologists
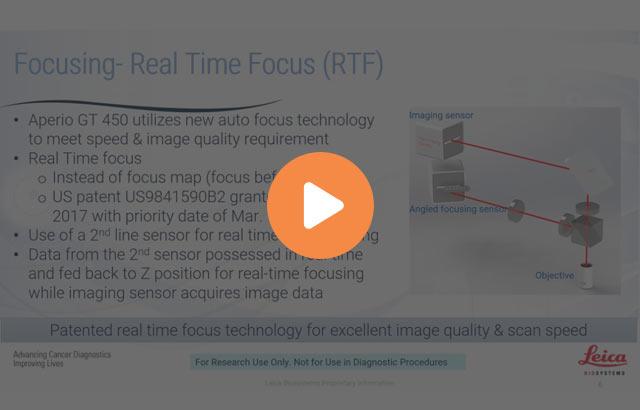
The benefits for pathologists includes excellent image quality, Leica optics combined with RTF plus line scanning, 40x scans with 0.26 micro millimeters per pixel resolution, German craftsmanship, the objective custom made by Leica Biosystems in Germany. It utilizes a real-time focused technology. And the confidence, the system is calibrated during every scan of the slide. It's 99.5% accurate tissue finder and has an automated image quality check.
This experience is further enhanced by calibrated viewing experience. That includes calibrated 2K monitors, the workstations loaded with the proprietary internal color consortium profile, which enables the monitor color calibration.
Color Calibration
This slide shows you how the color calibration works. It calibrates between the scanner and the monitor using an ICC color management method. The ICC profiles converts between the RGB from the scanner and the color. It creates a unique ICC profile for the scanner and the display monitor for accurate color. The output color measures on the display matching the spectral color on the stain.
Image Quality Considerations
Rather than relying solely on the conventional microscope and glass slides, the Aperio GT 450 allows you to clearly identify the cells and the morphologic features of both cancer and normal cells. It delivers an excellent image quality.
It allows us to see architectural details, including the rete ridges and dermal papilla of the skin and the villi of small intestine.
It also allows us to see a columnar cell, cilia, details of cellular border and intracellular bridges between the squamous cell.
And my personal favorite, as you can see here, it allows a crisp evaluation of a nuclear details, including nuclear membrane and nucleoli.
The Aperio GT 450 resolution and image quality brings a whole new level to the digital microscopy. You can see the details here very clearly.
In addition to excellent crisp images we saw before, GT 450 can also compensate for an occasional artifact or fault that can be present on the slide. Note the details of the IHC stain here presented here and the other on both sides of the artifact.
Image Quality Measurement
Just a few small details on how we evaluated the quality of the Aperio GT 450. Each of the three participating pathologies viewed and score image quality for 155 images first generated with a Aperio AT2 and later generated with the Aperio GT 450. The images were viewed using Aperio viewing station running the Aperio eSlideManager and there was a minimum of a two-week washout period between the reads and the images generated by both machines.
Image quality was assessed by each participating pathologist using a quadrant-based coloring criteria shown in figure one. For example, a score of four was recorded. If he or she can easily make a decision with confidence and could say that it was the same quality as the microscope or even better.
In this slide you can see that the scores between the three pathologists were very consistent.
From Lab to Pathology
In this final slide, you can see the workflow from the pathology lab, through IT room, IT managers office, and directly to the Pathologist office where we can view these images on our monitors and make a correct and accurate diagnosis. I believe that the GT 450 can deliver the image quality and speed that is essential for any modern laboratories.
At this point, we will proceed with Q&A session and I will address any comments. Thank you.
About the presenters

Dr. Chizhevsky is the Senior Hematopathologist and Medical Director, Flow Cytometry, NeoGenomics Laboratories, Inc. He is a member of the College of American Pathologists, American Society for Clinical Pathology and International Clinical Cytometry Society. Dr.Chizhevsky has authored many publications in medical journals.

Julie Broccardo is the Director of Pathology Operations for NeoGenomics Laboratories, Inc. She is responsible for managing multi-site operations, including the Ft. Meyers, Houston, Carlsbad, and Aliso Viejo laboratories. Julie is passionate about developing her staff and working on initiatives to build and develop a more efficient lab.
Related Content
El contenido de Leica Biosystems Knowledge Pathway está sujeto a las condiciones de uso del sitio web de Leica Biosystems, disponibles en: Aviso legal.. El contenido, incluidos los webinars o seminarios web, los recursos de formación y los materiales relacionados, está destinado a proporcionar información general sobre temas concretos de interés para los profesionales de la salud y no está destinado a ser, ni debe interpretarse como asesoramiento médico, normativo o jurídico. Los puntos de vista y opiniones expresados en cualquier contenido de terceros reflejan los puntos de vista y opiniones personales de los ponentes/autores y no representan ni reflejan necesariamente los puntos de vista ni opiniones de Leica Biosystems, sus empleados o sus agentes. Cualquier enlace incluido en el contenido que proporcione acceso a recursos o contenido de terceros se proporciona únicamente por comodidad.
Para el uso de cualquier producto, debe consultarse la documentación correspondiente del producto, incluidas las guías de información, los prospectos y los manuales de funcionamiento.
Copyright © 2026 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.