
Reducing Batch Size to Improve Slide Turnaround Time

The Leica Biosystems Process and Solutions Optimization team has partnered with Queen Elizabeth Hospital to examine how to optimize their processes to reduce turn-around-time. QEH is most interested in having slides assigned to their Pathologists earlier in the day. The Process and Solutions Optimization team identified a few changes to help recognize and monitor their goals.
Following a workflow assessment, it was determined that the large batch sizes in use were causing delays and creating large wait times in several steps of the Histology processes. It was recommended that the laboratory reduce the batch sizes from embedding to case assembly in order to decrease the wait times and result in slides being completed earlier.
Figure 1 shows the comparison between large batches and small batches. Using a large batch takes more time to complete, whereas the smaller batch allows work to move to the next step in the process more quickly.
Figure 1.
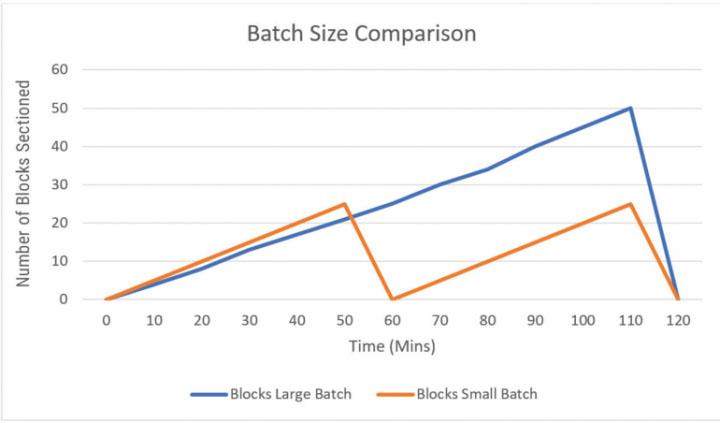
Figure 2 shows the average daily number of slides ready for QC by 7AM (embedding starts daily at 5AM). The Assessment data was collected during the on-site assessment in March 2018. The Pre-Batching data was collected by QEH staff in April 2018. The Post-Batching data was collected by QEH staff after the switch to smaller batch sizes in May 2018. That resulted in a 177% improvement in slides ready for QC compared to the assessment.
Figure 2.
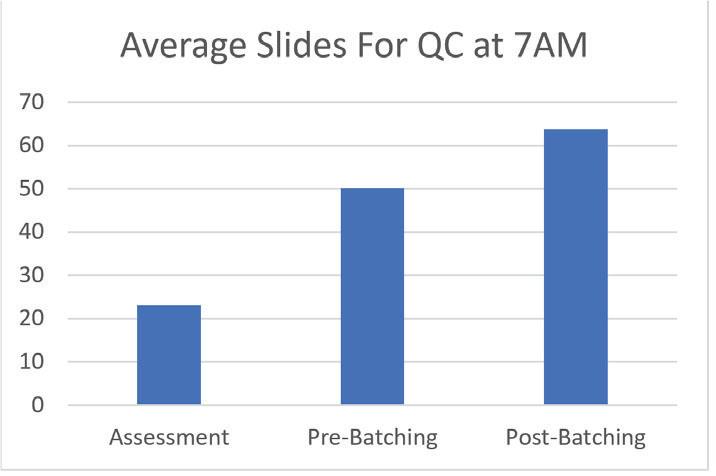
During the initial assessment in March 2018, the Pathologists had complained that on most days they did not receive any slides until after 10AM. In the initial month after the switch in batch sizes, slides were ready for the Pathologist on average by 8:09AM.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
Want to see how the Leica Process and Solutions Optimization team can help improve your lab's workflow?
About the presenter

David Newell has been working in the field of Laboratory Medicine for over 20 years. He has experience working on the Laboratory Informatics Implementation team, leading installations across North America. Before joining Leica Biosystems, he served in several roles at a large reference laboratory in Arizona, including over eight years as a Histotechnologist. He has experience managing an Anatomic Pathology Department for a level 1 trauma center and a dermatology laboratory. David holds a Bachelor of Science in Kinesiology and Master of Business Administration and Histologic Technician (HT) certification through the American Society for Clinical Pathology (ASCP). He received his Six Sigma and Lean training through Quest Diagnostics in 2002 and 2010, respectively.
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.
