Effects of Fixation and Tissue Processing on Immunocytochemistry
One of the first uses of formaldehyde as a fixative for tissue specimens was accidentally discovered by a Dr. F.Blum towards the end of the nineteenth century. Dr. Blum was not a Pathologist, but he did have an interest in bacteriology. When asked by a chemical company to assess a new commercial product as an antiseptic (a solution of formaldehyde) he diluted it 1:10 to produce a 4% solution and discovered that the solution was a good, but slow-acting germicide. He also noted that when the solution remained on his fingers for a short period of time, they became stiff. This stiffness was the same type of stiffness that he had experienced with other fixatives such as alcohol. Based on Blum’s observations this 4% solution has remained the percentage recommended for formaldehyde fixation and has not been challenged despite the well documented detrimental effects formalin fixation has on tissue immunoreactivity!
However, from the standpoint of consistency of microscopic appearance following paraffin processing and hematoxylin and eosin staining formalin fixation has been the choice of pathologists for the last century. Pathologists are trained to interpret formalin fixed tissue sections and departures from this cause changes to tissue morphology and can cause problems in diagnosis. With the increased use of immunocytochemistry in diagnosis, the problem with decreased immunorecognition in formalin fixed paraffin processed tissues might have led to a change in the choice of fixative, if antigen retrieval techniques had not been developed.
Immunoreactivity
The main target of immunocytochemists is to devise protocols that produce the greatest sensitivity without compromising specificity. The quality of primary antibodies has an enormous effect on specificity, but tissue fixation has the greatest impact on sensitivity of immunocytochemical methods.
Fixation alone does not characteristically cause a loss of immunorecognition of tissue antigens. Immunorecognition for some antigens is lost after specific combinations of fixation, tissue processing and paraffin embedding. An antigen demonstrated with a fixative used on frozen sections may not be demonstrable after using the same fixative plus subsequent processing, paraffin embedding and preparation of paraffin sections even when the same antibody is used in both staining procedures.
Formaldehyde denatures tissue macromolecules, thus making tissue proteins that comprise the majority of tissue antigens inaccessible to the primary antibodies used in immuno-cytochemistry (antigen masking). It has been shown that aldehyde induced cross linkages could be reversed by heating at high temperatures or by treating with strong alkalis. Shi et al1 applied these techniques to tissue sections and demonstrated that pretreatment of sections immersed in heavy metal solutions and heated in a microwave oven significantly increases the sensitivity of the immunocytochemical technique. They named their technique the “antigen retrieval” method and since then a number of other heating devices eg pressure cooker, autoclave, steamer and water bath have been reported to achieve similar effects. The term “heat induced epitope retrieval”, or HIER has become a widely accepted descriptive term. Prior to HIER the use of proteolytic enzymes2 was the most popular approach to reversing the masking effects of formalin fixation (EIER). The choice of protease, its concentration and its duration of digestion are largely empirical. It is however important to optimize the use of the enzyme of choice, rather than to use a broad range of enzymes.
Summary
The foundation of all good histological preparations is adequate fixation and good tissue processing. Recent demands on laboratories to decrease specimen turnaround times for histological diagnosis frequently result in the time a specimen should be allowed to fix in formalin, being curtailed.
Poor or inadequate fixation leads to poor paraffin embedding which leads to the production of poor quality paraffin sections. Sections cut from poorly processed tissue blocks show poor resistance to the rigors of antigen retrieval techniques and are easily lost. Morphology is impaired and antigens may be lost or become diffuse.
Despite the problems associated with formalin fixation the importance of this aspect of basic histological technique should never be compromised if high quality immunostaining is to be achieved.
Figures
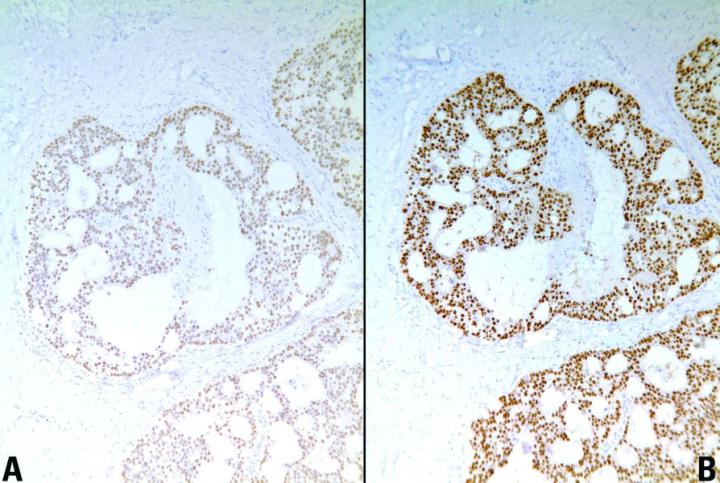
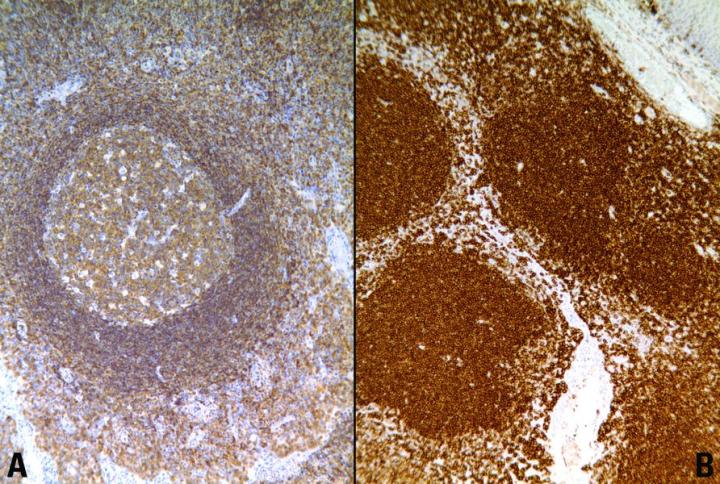
Key Factors for Enzyme Digestion
The key factors for enzyme digestion are:
- Temperature
- pH
- Enzyme concentration
- Duration of digestion
For optimum immunocytochemical staining, digestion time is critical and depends on the duration of fixation in formaldehyde. Tissues fixed in formalin for long periods usually require prolonged exposure to proteolytic enzyme. It may be found that digestion time varies because of batch- to- batch variation in enzyme activity. Another pitfall with enzymatic antigen retrieval is that poorly fixed tissues are easily over digested with resulting loss of morphological detail.
Where fixation and paraffin processing schedules are not known, as in the case of referred tissue blocks and sections, it may be necessary to perform a range of digestion times in order to achieve optimal immunocytochemical results.
There are a number of advantages and disadvantages of HIER. The main advantage is that heating times to retrieve antigens tend to be standard, regardless of the duration of fixation. Other advantages include:
- Increased intensity of staining
- Demonstration of antigens not normally demonstrable in formalin fixed tissue
- Production of consistent, reliable, high quality immunocytochemical staining of formalin sensitive tissue antigens.
Standardization of Basic Procedures
Standardization of the basic procedures used in histopathology such as fixation, tissue processing and the treatment of tissue sections prior to immunostaining should make a major contribution in improving laboratory performance. However, tissue processing is far from standardized. The formulation of the fixative used i.e. NBF, formal saline, or the use of other solutions of formalin such as formal calcium, has traditionally been left to each individual laboratory. The same is true of the choice of tissue processing reagents and duration of tissue processing. This lack of standardization of the fundamental processes of histology culminates in the preparation of a unique preparation – a tissue block that is unique and characteristic of the laboratory that produced it. Subsequently sections cut from such a block will provide substrates for immunohistochemical investigations that may be vastly dissimilar to sections cut from material fixed, processed and sectioned elsewhere. In dealing with such material it is essential that laboratory personnel understand the problems and pitfalls associated with referred material and must be fully aware of the established ways of manipulating protocols in order to produce high quality immunostaining.
Successful immunocytochemistry may be seen as the correct integration of several technical parameters. The aim of immuno-cytochemistry is to achieve reproducible and consistent demonstration of antigens with the minimum of background staining whilst preserving the integrity of tissue architecture. Fixation and subsequent paraffin processing is an essential consideration.
Adequate and appropriate fixation is the cornerstone of all histological and immunocytochemical preparations. Ideal fixation is a balance between good morphology and good antigenicity. Poor fixation, or delay in fixation, causes loss of antigenicity or diffusion of antigens into the surrounding tissues. Poorly fixed blocks do not process to paraffin adequately. Alcohol used in the tissue processing dehydrating steps is an excellent fixative and an excellent dehydrating agent. However if asked to perform both as a fixative and as a dehydrating agent, as with poorly fixed tissue blocks, it fails to achieve either and so a block which is poorly processed and therefore inadequately impregnated with wax is the end product. However in cases such as these all may not be lost as reprocessing may be carried out and reasonable immunocytochemical staining can be achieved for most antigens.
The College of American Pathologists (ASCO- CAP guidelines) has tried to address standardization of fixation in breast tissue in the USA. Their recommendations are that tissue be fixed for a minimum of 6 hours and a maximum of 48 hours in NBF3. The neutral buffered formalin must be less than 1 month old. Fixed tissues that cannot be processed immediately to paraffin should be stored in 70% alcohol until processed. To date in the UK no official recommendations regarding the type of fixative or duration of fixation exist. A study carried out by Goldstein et al 20034 demonstrated that false negative estrogen receptor immunocytochemistry could result if breast biopsies, needle core biopsies and resection specimens, are fixed for less than 6-8 hours. It is therefore generally thought best to fix tissues overnight rather than to rush the specimen through paraffin processing in the same working day. The amount of fixative used should ideally be 15-20 times the bulk of the tissue to be fixed. Tissues selected for sectioning should be sufficiently thin to be adequately fixed in a reasonable time. The bulk of the tissue determines the volume of fixative required and the thickness of the specimen will determine the speed of fixation.
Optimal Dilution of Primary Antibody
The optimum dilution of primary antibody for immunocyto-chemistry is the concentration of the primary antibody that gives the optimal specific staining with the least amount of background staining. The optimal dilution will depend on the type and duration of fixation. Therefore, it is of the utmost importance for individual laboratories to find the dilution (titre) of primary antibody that gives the best result using their preferred immunocytochemical detection system. Thus, it is rare that individual laboratories use identical dilutions of primary antibodies even though they may use identical demonstration immunocytochemical methodology.
References
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin embedded tissue: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741-748 1991.
- Huang SN, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest 35:383-390 1976.
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred CD, Cote RJ et al. American Society of Clinical Oncology/College of American Pathologist's guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Clin Oncol. Jan 1;25(1):118-45. 2007.
- Goldstein NS, Ferkowicz MT, Odish E, Mani A, Hastah F. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol 120:86-93 2003.
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.