Leica Biosystems is a global leader in anatomic pathology, offering the most comprehensive portfolio of solutions and one of the largest global installed bases of IHC instruments. We partner with leading pharmaceutical companies to develop and quickly integrate innovative diagnostics into anatomic pathology laboratories worldwide.
Image

Scale Access to Therapies Faster with Our Broad CDx Capabilities
Image
Flexible CDx Development
- Develop fit-for-purpose assays leveraging our experience from 1,300 IVDs globally, including IVDR compliance for multiple IHC products including CDx.
- Optimize timelines with flexible and modular development & robust QMS
- Advance diagnostic programs from research to clinical to commercial via our CAP CLIA lab*
Image
Broad Research-to-Clinical Portfolio
- Access to 11K antibodies through Leica and Danaher’s Abcam
- Expand to multi-modal CDx via access to other Danaher companies: Beckman Coulter and Cepheid
- Reduce variability by leveraging our leadership in digital pathology and AI partnerships
Image

Fast Commercial Scale-Up
- Enable patient access to novel therapies via our 8,000+ IHC instruments & presence in all top US cancer centers
- Accelerate adoption by partnering with our 1,300+ commercial associates
- Ensure CDx continuity via our robust supply chain serving 100+ countries
*CAP CLIA certification pending
Supported by proven project management experience |
|
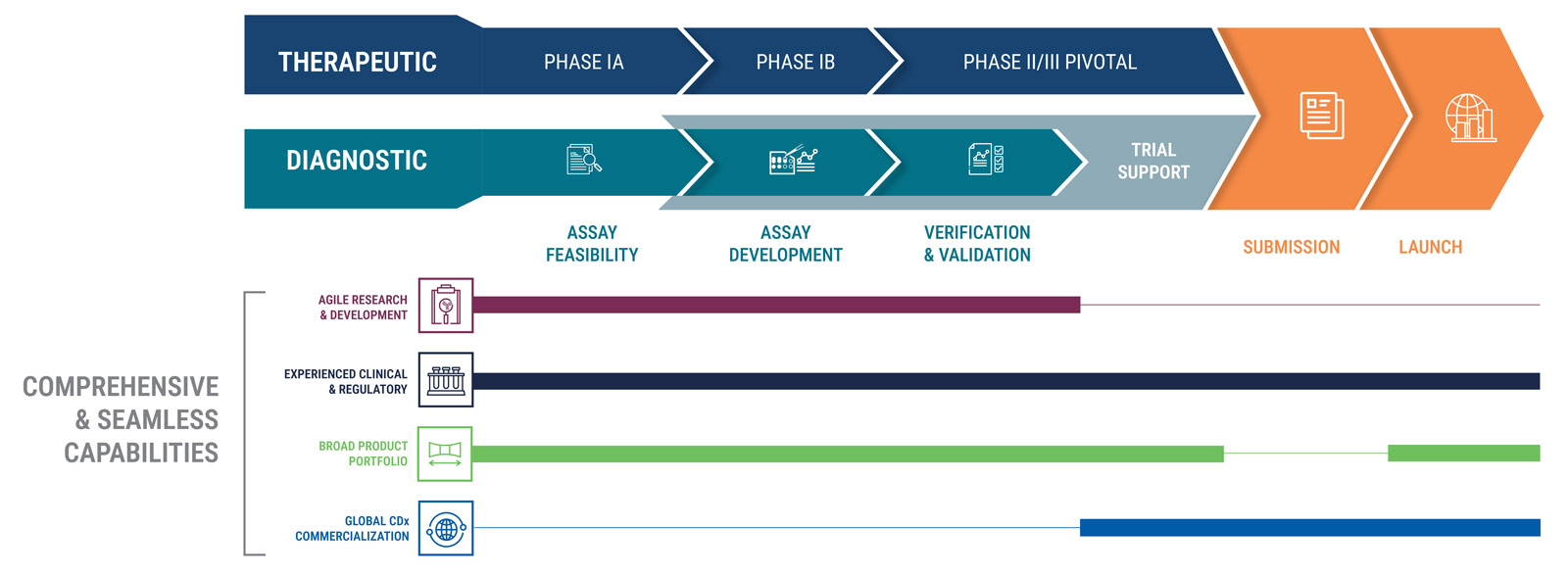
Comprehensive and Seamless CDx Co-Development Process
What's New
Leica Biosystems Opens New Center for Enabling Precision Medicine to Accelerate Companion Diagnostic Development
Leica Biosystems opened the doors of its new research and development facility in Newcastle Upon Tyne, UK, to accelerate the development of Companion Diagnostics (CDx). CDx tests enable patients to receive targeted therapies to better treat their disease.