Dissection of the melanoma microenvironment via high-throughput multiple iterative labeling by antibody neodeposition (MILAN)


In this webinar, Prof. Francesca Bosisio and Asier Antoranz, assistant professor and post doc bioinformatician at University of Leuven, provide a practical example of what digital pathology can achieve, illustrating how high-dimensional single-cell multiplex analysis using multiple iterative labelling by antibody neodeposition (the MILAN technique) can characterize the immune landscape in primary melanoma.
Learning Objectives
- Understand the basic principles of the MILAN method
- Briefly explore the state-of-the-art technology available for spatial tissue multiplexing
- Discover a computational approach to multiplexed image analysis
Webinar Transcription
Hello, and a very warm welcome to the second webinar in our Multiplexing Masterclass program, titled Dissection of the Melanoma Microenvironment via High-Throughput Multiple Iterative Labeling by Antibody neodeposition. My name is Charlie Carter, and I'll be moderating today's presentation. And I'm delighted to be joined by Francesca Maria Bosisio and Asier Antoranz of the University of Leuven, who will be continuing our Multiplexing Masterclass today. Please feel free to submit your questions for the Q&A session at the left of your screen at any time during the webinar. And without further delay, I'll hand over to Francesca and Asier.
Thank you for the introduction. As anticipated, I am Professor Francesca Maria Bodivio from the University of Leuven.
The Melanoma Microenvironment Dissected Through MILAN
I'm Asier Antoranz, a postdoctoral researcher from the University of Leuven.
Today we're going to give you an example of our research in the melanoma field using our multiplexing technique. We’re going to first introduce you to the field of tissue multiplexing through a short introduction about the single-cell analysis in science. Then we will see how multiplexing can bring us to the next generation pathology. And we want also to give you an overview of the potential challenges when selecting a multiplexing technique for your research. And finally, we are going to talk about our last paper, where we present how tissue multiplexing can be applied in research.
Single Cell Analysis in Science
We will start from single-cell analysis in science. It was 2017 when Nature published an issue all dedicated to single-cell analysis. Some of the sentences in that issue already defined the direction in which this field must go. First, it was already clear from the beginning that as multiple technologies to study single cell are developed, we would have also required sophisticated analytical tools to tame and make sense of the results. And these tools could be applied not only to dissect the identity of tumor cell subpopulations, but also to identify dozens of types and functions of new cells in the tumor and macroenvironment. Since the beginning, it was already said that not only we have to try to collect all the cells within a tissue or a region of interest, but that the success of these single-cell technologies will depend on the extent to which we could preserve the states of the cells and the original composition of the tissue. In fact, we know that most of the single-cell methods available are based on tissue dissociation.
The tissue gets destroyed and the cells isolated before the analysis. And this can also change their cell state. In recent years, however, methods have been developed to finally achieve the goal of preserving the tissue. And this has given us the chance not only to study the cytometry of the tissue, that is its basic composition in terms of cells, but also the sociology of the tissue that it's based on using spatial distribution to make assumptions between cell interactions. And transferring the single cell analysis on a tissue, it means to set the basis for next generation pathology to happen.
Pathology (R)evolution
Pathologists’ position that in between the patient and the correct treatment of this disease. And medicine is now trying to move towards personalized treatment. But is pathology ready for this? Pathology has been evolving dramatically in the last 10 years. It's an old discipline that originally was based on the recognition of tissue morphology based on a simple H&E staining. It saw its first revolution in the '80s with the introduction of immunohistochemistry that was based on the simple principle of using antibodies conjugated with detection systems to recognize proteins. After these other two revolutions emerged, the introduction of molecular techniques in pathology, and most recently, the introduction of artificial intelligence as a support to pathologies.
Matching Patients to Treatments
Even though in the clinics, we are still using the same immunohistochemistry that was used in the '80s, in the last years, immunohistochemistry has further reviewed. Currently, pathology uses in the clinical work conventional immunohistochemistry that allows to stain one marker at a time. Therefore, the evaluation of multiple biomarkers can only be done on serious sections. One section cut after the other. This obviously does not allow an evaluation of these biomarkers at the single cell level, given that the same cells are not present in consecutive sections. We easily lose them due to the cutting of the material. And to move towards next generation pathology, it needs to characterize every single cell in the tissue of a patient to achieve a real personalized treatment, and that must be guided by the type of cells present in that tissue and by the markers that they are expressing at single cell level.
Matching Patients to Treatments by Multiplex Analysis
This means that it might be necessary to stain for more than 50 markers at the same time for one single section, and this is where multiplexing becomes fundamental for next-generation pathology. But tissue multiplexing is not just a repetition of multiple conventional immunohistochemistry. It's a much more complex process. And there are some challenges that anyone who wants to start doing tissue multiplexing should be aware of.
Challenge 1: Wet Lab - Which Method to Choose?
The first challenge is about which wet lab technique method to choose. A lot of different multiplexing techniques have been brought to the market, but the choice of which method to implement in your lab is guided by your own research project. First, you need to consider the type of samples that you are going to use. There are some methods that work with formalin fixed paraffin-embedded material and others that work better on proven material. Then you will also have to choose between different ways to detect and remove the signal. There are methods that remove the antibodies from the section, methods that only bleach your signal away, but the antibody stays. And these first two approaches, the stripping and the quenching of the signal, are characteristic of cycling methods that need to be repeated several times before completing your whole experiment.
On the other hand, there are methods that guarantee an all-at-once acquisition by dropping a cocktail of all your antibodies on the tissue section at once. And the signal removal method is important, because it will determine if you can preserve your tissue slide to eventually add more markers in a second phase, because some methods will destroy your tissue while others won't. Also, the antibodies will be different depending on your method. Tripping and quenching use regular antibodies, while all-at-once acquisitions will need engineered antibodies that might be conjugated with heavy metals or nucleotidic barcoding. And finally, the secondary removal method will also influence your speed and scalability. That depends not only on how many micrometers of tissue you can scan per minute, but also by the number of slides that you will manage to stain in each cycle.
The scalability is also influenced by the degree of automation of the method. The method can be fully or partially automated, or can be offered as a service, providing you directly with the images. The speed of the method will influence the extension of the area that you can apply. Not all the methods will allow you to apply the whole slide in a reasonable time, but some will force you to focus on a smaller region of interest. The level of automatization will also define the type of instrumentation that you will have to buy. Fully automated methods often come as closed systems, while partially automated ones involve separated components that are already present in most of the research labs. Moreover, the type of antibodies that the method uses influences the flexibility of the methods. All these methods allow you to build fixed panels of validated antibodies. But when it comes to introducing new antibodies, the simpler the technology is, the easier will be the customization.
For example, not all the antibodies available on the market can be easily communicated. Then the signal removal method, the type of antibodies that you use and the flexibility of your system, will determine the maximum number of markers that you will be able to plex. There are methods with low throughput, where less than 10 markers can be multiplexed, and methods with high throughput that can achieve at least 20 markers on a single section. And finally, the types of antibodies that you will use and the type of instrumentation that you will need to buy will determine the final cost of the method, given that engineered antibodies enclosed systems are normally significantly more expensive than regular antibodies and machines that are already used in immunohistochemistry labs.
Technical Comparison of Multiplex IHC Methods (Antibody Based)
As I said at the beginning, there are different methods that are available on the market. These methods, more than being one against each other, are complementary because one method can fit a research project, but not another method. Therefore, the only criterion that can help you choose which method is best for you are the type of experiment, so your material, how many slides you must stain. If you can go for a region of interest, it's better to have whole slides for your research question. The number of markers that you want to plex and how much time you need to perform the whole experiment. Then, obviously, your funds. It depends on how much funding you can invest in acquiring all the instrumentation that is needed, and how much you can sustain in the longer term the cost of the antibodies and the reagents.
Then you must consider that there is also time needed for validating the panels and to train your technician or your students to use a certain method. Finally, it's important the type of expertise that you have on your team, because I would like to stress that tissue multiplexing is not the simple sum of single immunohistochemistry. It's a level of complexity higher. You have also to consider that most of the wet lab methods are not paired with a system to analyze the data. You will need not only good researchers, good technicians, clinicians to select your patients and pathologists to interpret the staining, but also experts in image analysis and bioinformaticians for the downstream analysis.
This is when we come to the second challenge. What am I going to do with the images that I generate? You must consider that a small project with around ten markers to be staying in ten patients in ten samples will generate 100 images. A medium-sized project with thirty markers and fifty samples will generate 1,500 images. The largest project that is what you normally go for in research will generate 9,000 images. And this is why multiplexing is not anymore for visual inspection or to create appealing images, but it's a method to be analyzed quantitatively with software that specifically is developed for image analysis. And there is some software that is available on the market, but the expertise of the person that uses the software is very important. I will leave now my postdoc here to speak about some of the steps that you may encounter when you want to process, pre-process your images for the analysis and the potential that the analysis has.
Challenge 2: Dry Lab - What Am I Going to do with the Images?
Thank you, Francesca. Professor Asier mentioned that there is a strong need not only for wet lab people, but also you need experts in image analysis and bioinformatician. And this need is mainly due because the number and the type of steps involved in computational analysis depends on the selected wet lab technique. Each technique has its own particularities and will require to fine-tune the pipeline. For example, if you have a cyclic method, the images acquired in sequential rounds are not necessarily aligned and need to be registered. Registration is just a computational technique that aims at the pixel-level overlapping between a reference image and a moving image. Secondly, one of the challenges of working with immunofluorescence is that it requires the excitation of the tissue with a specific wavelength to obtain a signal. However, upon excitation, different molecules in the tissue emit light, like famously blood. Therefore, not all the acquired signal comes from the market of interest. This issue is known as tissue autofluorescence. Some techniques use wet lab approaches to tackle tissue to fluorescence like for example the application of Sudan black staining while other methods use baseline images with without antibodies to be subtracted from the measured signal and obtain the true signal.
Then the next step involves the identification of all the single cells in your images. This is known as cell segmentation, and there are many different methods in the literature that tackle this problem. Most of these methods will focus on the segmentation of the nuclei of the cells, which comes from DAPI stainings, and are based on deep learning approaches. Well, deep learning approaches require a huge volume of data to be trained, so you will have to also annotate your images. Then the last step of image analysis consists of extracting quantitative data from the cells identified within the segmentation. The type of data and features that can be extracted is normally broadly classified in three categories. You can have morphological features, like, for example, the size, the cell shape, and so on. Topological features, like XY coordinates, or, well, if you have 3D tissue, you also can include the Z coordinate, and intensity-based features, like the average or the median, that collect the expression of each measured marker. And from this point onwards, your analysis will not be focused anymore on images, but rather on a data matrix that represents those images. And at this point, well, the type of analysis that you want to perform depends on the biological questions that you want to answer.
Generally, the baseline is that you want to identify all the different cell phenotypes that are present in your tissue. For this, the most common approach is clustering. Clustering is a computational technique that aims at grouping cells with similar expression profiles together. There are a lot of different clustering methods available in the literature for single cell data. And well, one of the drawbacks is that each of them will produce a different result. And that's why clustering is normally coupled with the manual annotation of the clusters to non-cell phenotypes. And this is done by expert pathologists who know the phenotypes that should be on the tissue. Manual annotation of the clusters also allows to digitally reconstruct the tissue and adding another level of comprehension to the analysis.
Finally, one of the main benefits of using wet lab techniques that preserve the topology of the tissue is that you can extract information about tissue sociology. That is, you want to infer which cell phenotypes are interacting or avoiding each other. A common approach to answer this is neighborhood analysis. In neighborhood analysis, well, not something that you can do, it's imagined that you have two types of cells, A and B. You can count the number of cells of type A that are closer than a certain distance to the cell type B. Then you randomize the tissue by, for example, shuffling all the annotations that you have done and repeat the process. If the counts that you obtain in the real tissue are more than those that you obtain in the randomized case, you assume that the cells are interacting with each other. However, if the observed counts are less than your random counts, you can assume that the cells are avoiding each other. Then you can repeat this same process for all the different cell phenotypes that you find in your tissue. You can build this social network that summarizes information about cell-cell interactions.
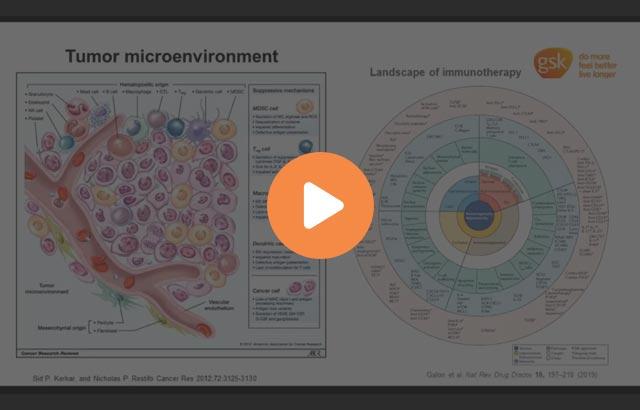
Immuno-Oncology
Talking about the application of tissue multiplexing, immuno-oncology represents an ideal field. Not only has pathology been evolving these years, but also oncological treatment has radically changed. I will make an example of melanoma because it's my field of research. In the last 10 years, we have gone from the one-fits-all chemotherapeutic treatment with dacarbazine to a first attempt at personalized therapy that is represented by targeted therapy. Targeted therapy was introduced around 10 years ago and is based on molecular recognition of specific mutations that can be targeted by specific drugs. At the same time, another type of treatment was being developed, and this is checkpoint inhibitor therapy, a type of immunotherapy that is based on blocking the immunitary system, sorry, the immunitary breaks that prevents our immune system from being hyperactivated.
Checkpoint inhibitors therapy represented a revolution in melanoma treatment, because while targeted therapy encountered the development of resistance, determined the relapse of the melanoma, after a certain period of treatment, immunotherapy can bring two responses that have a very long duration in time. But these responding patients that are the patients that we like to see are only a small percentage of the total. We have also patients that we don't like to see, and these are the ones that do not respond to immunotherapy. Immunotherapy, in fact, is not only good news, because only around 30% of the patients respond. And moreover, these therapies are burdened by immune-related adverse events, and they are very costly for society. Therefore, we need to find predictive biomarkers to give these drugs only to the patients that will benefit from them and therefore achieving a real personalized treatment.
Some biomarkers have been identified in the literature, but none of these have found the required success to be implemented in the clinics. On the side of pathology, single-plex markers like PD-L1 staining have been implemented, but still with very sufficient predictive power. And this is where next-generation pathology can become handy, not only to evaluate panels of multiple biomarkers at the same time, but also to integrate this information with the cell types that express them and their spatial distribution.
We will give you now an example talking about one of our last papers in which we have investigated the melanoma microenvironment using our multiplex technique that is called MILAN. MILAN, this is Multiplex Staining by Sequential Immunostaining and Antibody Removal, is a cyclic method based on the stripping of the antibodies. It employs regular commercially available antibodies applied three at the same time on one tissue section. The stainings are done with the Leica BOND RX and it is therefore a semi-automated method. The images are acquired with a scanner and then the antibodies are stripped, and the cycle is repeated. It works on formalin-fixed paraffin embedded material, and you can acquire host-like images and you can store the sections to make additional stainings in a second phase of your research.
Research Question
Our research questions started from the fact that the lymphocytes that infiltrate the melanoma are classified on disease of morphology. They are brisk if they are diffusely present in the tumor, non-brisk if they are concentrated in certain areas, but are absent in other areas of the tumor, and absent if they don't get in contact with melanoma-sensitive groups. The brisk infiltrates are correlated with better survival. But brisk means active, lively. But it's based on a morphological definition. And in truth, we cannot say with morphology if a lymphocyte is active or not. We don't know if the lymphocytes in brisk infiltrates are active in comparison with the lymphocytes in the non-brisk infiltrates. And interestingly, it has also been described a group of tumors with brisk infiltrates that are associated with poor outcomes.
The only way to investigate the activation status of the lymphocytes is through tissue multiplexing because the expression of exhaustion markers starts from the beginning of the activation of the lymphocytes to prevent hyper immunity. Therefore, the combination of markers of activation and exhaustian will ultimately define the activity of the lymphocytes. With tissue multiplexing, we can also add multiple markers at single cell levels to identify all the different cell types that are present in our tissue. And thanks to the spatial information, we can perform neighborhood analysis between these different cell types to study the tissue sociology of the melanoma microenvironment.
To answer this question, we took 29 primary melanomas, six brisk and 23 non-brisk. We included them in a tissue microarray, and we performed the MILAN multiplexing to obtain information on the expression of 39 immune markers at single cell level. And then we proceeded with image analysis and data analysis.
At first, we focused on the functional analysis of the CD8-positive cytotoxic T lymphocyte population. To define the activation status of each one of these cells, we built a trajectory using the four different markers included in our panel that were related with activation and exhaustion. That is, we used CD69 and OX40 for activation, and LAG3 and TIM3 for exhaustion. Starting from the activation score of each individual cell, we extrapolated to the activation score of each individual tissue core. However, for each patient, we measured more than one core. The next step that we had to do was to further extrapolate the activation status of each core to the activation status of each patient.
Based on these results, we observed that the activation status of the lymphocytes in different areas of the same patient was homogeneous in most of the cases. Only very few patients presented areas of exhaustion and activation with the same melanoma. And we analyzed if this model of activation had also a prognostic impact. We could observe that in the same way that patients with skin filtrates have better survival, also patients with active lymphocytes have better survival. We further validated this model also in an independent data set.
To that end, we first calculated the gene expression profile of active and exhausted cytotoxic lymphocytes using our model of activation in a publicly available single-cell melanoma dataset. We use these profiles to expand the immune fingerprints present in CiberSort, which is a computational software that tries to predict the different immune populations, the percentages, starting from bulk RNA-Seq data. And we used this software to assign an activation-- well, we used the results of this software to assign an activation status to each patient present in the TCGA skin cutaneous melanoma dataset. After doing survival analysis on this data, we also saw the same as-- well, as for our data, validating that, indeed, active melanomas-- yeah, active melanomas have a better prognosis than exhausted melanomas.
We then correlated the activation status of the lymphocytes with different histopathological parameters, such as the histological subtypes, the mitosis, breast blood thickness, ulceration, and regression. We observed that the only one that was significantly correlated with activation was the presence of late regression, defined as an area of scarring with total disappearance of the melanoma cells. Early regression instead was characterized by a partial disappearance of the melanoma cells. associated with a dense inflammatory infiltrate was not associated with higher levels of activation. And this can be interpreted with early regression being just a stage and being too early to be sure that all the melanoma cells will be eliminated by the new response.
Then we move to the identification of all the different phenotypes present in our tissue samples. And as I mentioned before, because different clustering methods give different results, we follow a wisdom of the crowds type of approach and apply all three, well, in this case, three different clustering methods, which were PhenoGraph, k-means, and cluster X, and well then found consensus between the annotations given by each of the three clustering methods for each cell.
Okay, with this method, we could characterize all the inflammatory subpopulations present in our tissue course and evaluate if the brisk/non-brisk pattern and the activation status of the cells were significantly correlated to the immune cell composition of the tissue. In A, you see the general composition of the immuno-filtrates in all our patients. And then in B, you see only the significant differences between brisk and non-brisk cases. And in C, the significant differences between active transition and exhausted cases. We found that brisk cases were enriched in B cells, classical dendritic cells type 1 expressed in TIM3, macrophages, and proliferating macrophages, but also NK cells, allergic and proliferating cytotoxic T cells. And this represents for us a strange mixture of immune cells, because some are linked to immune stimulation and others with immune suppression.
When we looked at the activation status, instead, the enrichment was much more coherent. So active cases were enriched mainly in active cytotoxic T-cells and exhausted cases in exhausted cytotoxic T-cells, as expected. But in exhausted cases, we also had an enrichment of more proliferating melanoma cells, Tregs, and T-helpers with immune suppressive function. The last step was to investigate the relationships of these cell types through neighborhood analysis in brisk and non-brisk cases. This first histogram shows an approach of neighborhood analysis that is tailored to explore specifically the interactions between inflammatory cells and melanoma cells. In general, the many inflammatory cell subtypes in contact with melanoma cells were macrophages and epithelial cells. But in brisk cases, mostly active and in transition, cytotoxic T-cells were in contact with melanoma cells, while in non-brisk cases, proliferating and anergic cytotoxic Ts when in contact with the melanoma cells. So this is a good example of how tissue sociology can be definitely superior to the mere tissue cytometry, because through this, we could understand that even if the total amount of active cytotoxic T was not different between brisk and non-brisk cases, the general good prognosis of melanoma is a brisk pattern could be based on the fact that the cytotoxic T that are in contact with the melanoma cells at the moment in which the melanoma is taken away from the patient are still active in brisk cases. Consequently, the melanoma is still under immune control.
We performed neighborhood analysis also between all the other inflammatory cell subtypes that we identified, and we could describe the interactions specific to active cases that could support the presence of an active microenvironment, such as, for example, the ones between active T-helper cells and active cytotoxic T. On the other hand, the cell interaction specific for exhausted cases included more immunosuppressive interactions, such as between semi-exhausted E helpers and anergic T cells, or anergic, cytotoxic, and B cells that express interferon gamma, or Tin-3 positive macrophages and TCI in transition.
Looking at the brisk and non-brisk classification, we can first observe that the neighborhood analysis profile of the brisk cases was very similar to that of active cases, and that non-brisk cases show more cell interactions linked with immune suppression. But nevertheless, both categories were not totally overlapping with the active and exclusive plots but rather presented a mixture of immune-stimulating and immune-suppressive interactions. This data confirms once again that the morphological categories are functional heterogeneous, and this could explain why a small part of risk cases may help reduce. This study supports the fourth direction to introduce a multiplexed evaluation in the future in the clinics for prognostic stratification of the patients, and possibly for prediction of the response to immunotherapy. We are testing this loss hypothesis on a dataset of immunotherapy-treated patients right these days.
Take-home Message
In conclusion, in this paper, we have shown that the classification of primary melanomas based on the functional paradigm had an improved prognostic value when compared to the brisk classification. And we have shown a bioinformatics pipeline that, starting from common immunofluorescence staining, can transform the tissue into a digitalized image, which represents a starting point for multiple deeper levels of analysis. The ultimate take-home message of our presentation is that the tissue multiplexing applications are now on the market for next-generation pathology and advanced biomarker discovery. You must choose the most suitable wet lab method that fits your own research, and you don't have to underestimate the burden of the dry lab analysis that has to be done properly. Just one last slide. to thank, first, Leica, for giving us the possibility to give this presentation. And then our amazing team at the University of Leuven and the University Hospital of Leuven that support us in our everyday journey through multiplexing.
About the presenter

Dr. Francesca Bosisio is a dermatopathologist and professor at UZ/KU Leuven. She participated in the development of the MILAN method in collaboration with the University of Milan-Bicocca.

Related Content
Leica Biosystems content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2026 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.