
Benefits of Implementing a Specimen Tracking System (STS) in Anatomic Pathology
INTRODUCTION
Anatomic pathology has historically relied on manual systems of tracking and data collection. While many laboratory information systems ( LIS ) provide rudimentary data on cases, tissue blocks and slides, they fail to report data in such a way as to be useful in real-time decision-making. This is evident when trying to find a missing block or slide, or when trying to establish the best use of staffing for a particular time period of the day (AP volumes can widely fluctuate from day to day.) Bar coding and tracking can be used to capture and manage data as well as help histology create standard work in its processes. We believe these process changes realize efficiencies to aid the histology staff in completing the work in a standard and safe way.
MATERIALS AND METHODS
The original assessment for CPA Labs was taken in February of 2016. Certain data points were gathered to understand and estimate the impact of STS. By observing and timing certain histology activities, we can reasonably estimate the amount of time the average histotechnician in that lab spends doing that particular task. Each metric is a timed measurement done by the same observer, observing multiple individuals performing the same tasks.
For the post-assessment, the same tasks were observed and comparably timed, and the results compared below.
Volume Comparison

RESULTS
For the purposes of this study, we examine the impact of a specimen tracking system in time saved per case. All steps of the histology process that involve manual labeling (except affixing a label to a specimen container at accessioning or slide at microtomy) have been eliminated and are now automated.
In the accessioning area, in some cases, the process has changed remarkably, as CPA/Norton has standardized the Laboratory Information System ( LIS ) across all of its facilities.
The result of this change has been to have a single process of accessioning specimens with analogous data from facility to facility. In the initial assessment, only the process using the now current LIS was observed as the other processes were scheduled to be discontinued. Previously, all specimens were manually assigned accession numbers and grossing occurred simultaneously to or even prior to the accessioning process. In addition, case numbers were manually written on specimen containers and cassettes were printed by manually typing in the case number into the printer (approximately 17 seconds/case). Following the implementation of specimen tracking, cases are now accessioned into the LIS where a case number is generated and specimen labels and cassettes are automatically printed, resulting in the elimination of two manual steps.
At grossing, each cassette was visually confirmed to match and then the specimen was grossed. Now, the specimen container is scanned into the STS to ensure a match and then the cassette is scanned into a “virtual bucket” that has a bar code assigned to it.
Cassettes are placed in tissue processing baskets where they stay in formalin until a courier picks them up to be delivered to the main CPA laboratory facility. To accurately capture the cassette information being transported, the courier scans the virtual bucket barcode and the location bar code to pick up cassettes and then scans the bucket and the new location bar code to deliver the cassettes to histology. This is an additional step that was not previously performed, but it was a safety capability of the STS that was sought after, as nearly all the cassettes come to the lab via courier. Cassettes are then loaded onto the tissue processors to be processed to paraffin.
Once tissue cassettes have been processed, they are removed from the tissue processors and are taken to embedding. Cassettes are then scanned to record the step and tech in specimen tracking and are embedded according to standard practice. This was previously accomplished by embedding the block and then manually checking that block off a paper log.
From embedding, cassettes are moved to microtomy where, instead of handwriting slides and leaving their workstation to print labels out of the LIS (an extremely time-consuming process), they are scanned to produce slide labels at the bench. Each block is scanned just prior to sectioning. This single piece fl ow is necessary to avoid mislabeling slides.
From microtomy, slides are stained with routine H&E. Slides are then assembled to be distributed to pathologists. This process involves sorting slides by case number and then scanning slides into specimen tracking as distributed to the assigned pathologist.
LABOR ANALYSIS
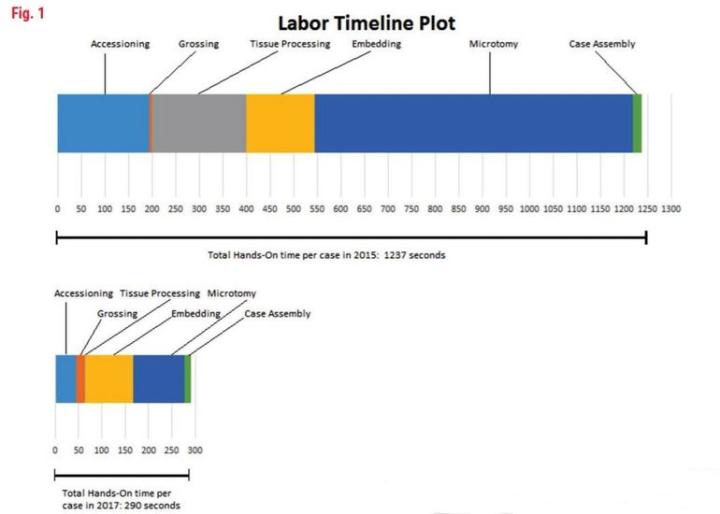
As you can see from Table 2, the breakdown of manual, “hands-on” tasks for each of the observed processes (accessioning, grossing, etc.) is shown in seconds per case. This was calculated by the observed number of seconds for the task, multiplied by the unit (block/slide per case) as calculated from that year’s volume. This yields a common measurement that can be consistent from year to year. The graph in Figure 1 shows a line plot of time from 2015 and 2017 and displays the remarkable difference in time per task with an overall 76.5% time savings in manual or “hands-on” tasks.
Table 2 - Hands-On Time Comparison
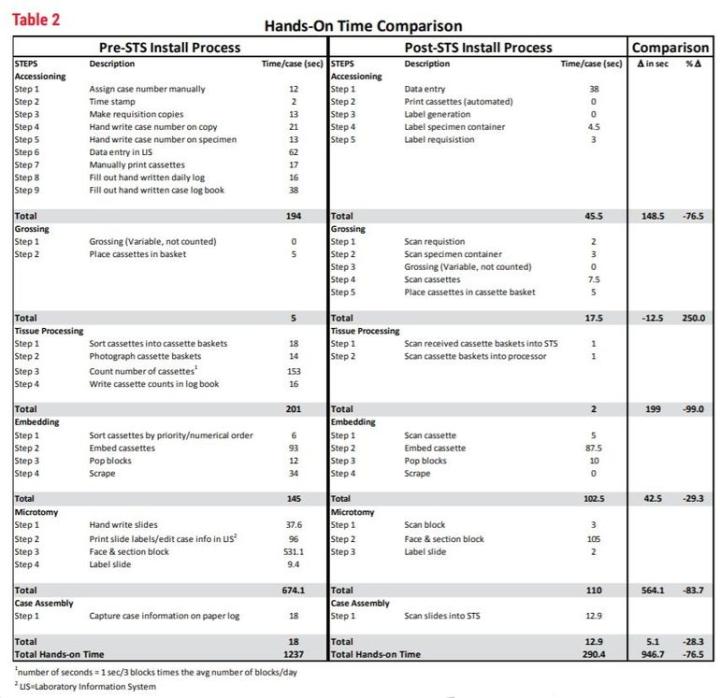
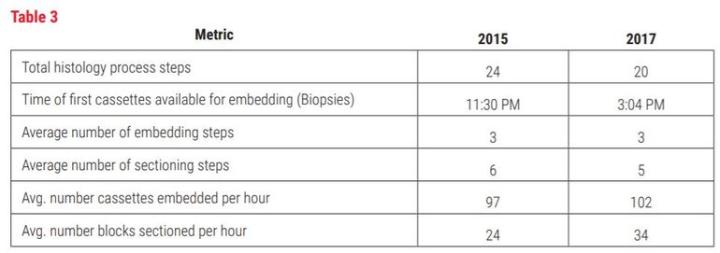
Table 3
Labor Time Plot
DISCUSSION
The implementation of STS shows measurable improvements in histology by the reduction in number of process steps and increase in productivity. The number of process steps has been reduced from 24 to 20. This may not seem like much, but the time impact of the steps eliminated reduced the “hands-on time” significantly by eliminating 4 major steps. The major steps eliminated were 1) the extremely time-consuming label generation process (approximately 3220 hours per year), 2) manually counting each block prior to tissue processing (approximately 10 hours per year), 3) elimination of photographs of baskets prior to tissue processing (approximately 4 hours per year) and 4) handwriting slides (approximately 134 hours per year). The other measurable improvement was seen in histology productivity in technical work. By streamlining tasks and eliminating paper, techs were able to add 5 blocks per hour average to embedding and 10 blocks per hour average in sectioning. We also see the amount of time to perform histology tasks (embedding, microtomy and case assembly) reduced from 837 seconds per case to 612 seconds per case, on average. This is a 27% reduction in hands-on time spent just in the histology lab.
The addition of STS along with other improvements (such as the LIS ) have enabled CPA Labs to save an average of nearly 16 minutes of technical (hands-on) time per case. The equivalent of 52.3 technical hours daily (946.7 seconds times the average number of cases/ day at 199 divided by 3600). This is greater than one full time equivalent per day. Through other improvements in process and products, the first available specimens (specimens able to be worked on in histology; see Table 3) are now at 3:04 PM rather than 11:30 PM.
Specimen tracking is needed in the Histology Laboratory. By removing the manual process of tracking the specimen, slides, and blocks it allows the technologists time to develop and improve their histologic techniques. The value of this time has increased as our seasoned technologists begin to retire, and our new graduates lack the tenure and time to be trained appropriately. Specimen tracking gives back this lost time for additional technical training by reducing the need of paper logs, phone calls, and time-consuming searches. The best aspect of Specimen tracking is the high degree of user-friendly applications with universal icons and text. The product was developed, and still in developmental upgrades, by histo-technologists and laboratorians who understand the needs of the laboratory better than the traditional software analysts and coders.
Disclaimer
This presentation was prepared by Shanna Brotzge Pollock, MSB, HTL (ASCP) QIHC, PA. Director Pathology - CPA Lab, Norton Healthcare. The presentation represents Shanna Brotzge Pollock’s views, and does not necessarily represent or reflect the views or opinions of Leica Biosystems. It is for educational purposes only and is not intended as regulatory or legal advice or as diagnostic advice for a particular patient or a particular disease or condition.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
El contenido de Leica Biosystems Knowledge Pathway está sujeto a las condiciones de uso del sitio web de Leica Biosystems, disponibles en: Aviso legal.. El contenido, incluidos los webinars o seminarios web, los recursos de formación y los materiales relacionados, está destinado a proporcionar información general sobre temas concretos de interés para los profesionales de la salud y no está destinado a ser, ni debe interpretarse como asesoramiento médico, normativo o jurídico. Los puntos de vista y opiniones expresados en cualquier contenido de terceros reflejan los puntos de vista y opiniones personales de los ponentes/autores y no representan ni reflejan necesariamente los puntos de vista ni opiniones de Leica Biosystems, sus empleados o sus agentes. Cualquier enlace incluido en el contenido que proporcione acceso a recursos o contenido de terceros se proporciona únicamente por comodidad.
Para el uso de cualquier producto, debe consultarse la documentación correspondiente del producto, incluidas las guías de información, los prospectos y los manuales de funcionamiento.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.