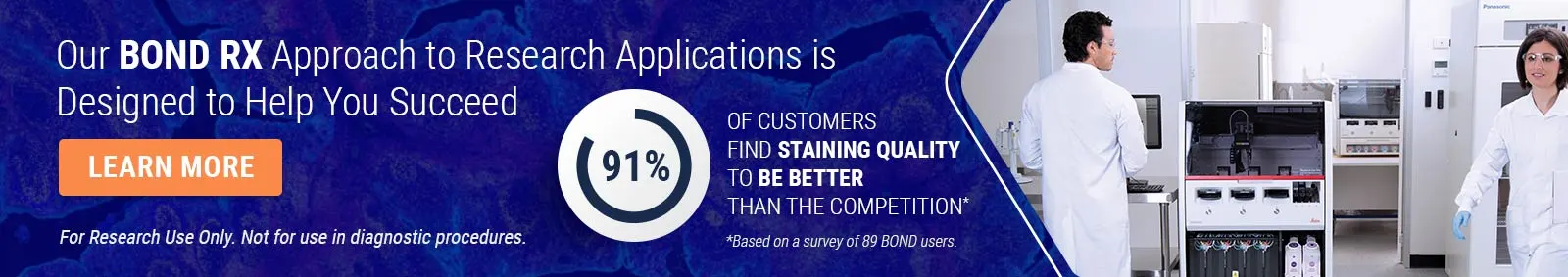
Introduction to Immuno Oncology

Learning Objectives
- History of Immuno-Oncology
- Types of Immunotherapy
- Immune Checkpoint Biomarker
Webinar Transcription
Introduction to immuno oncology
1. Introduction to Immuno-Oncology Michael Bates, MD Oncology Strategy, Medical and Scientific Affairs Cepheid Sunnyvale, CA Cepheid and Leica Biosystems are Danaher Companies
2. Overview • History of Immuno-Oncology • Types of Immunotherapy • Immunomodulatory agents • Personalized Cancer Vaccines • CAR-T cell therapy • Immune Checkpoint Inhibitors • Focus: Immune Checkpoint Biomarkers
3. Evolution of Cancer Immunotherapy • William Coley – 1890s surgeon • Coley’s toxin… • BCG, IFN-alpha, IL-2 • IL-2 applications in melanoma and RCC proved that advanced metastatic cancers can be controlled in some patients by stimulating T cells with cytokines • Critical development • CTLA-4 shown to be inhibitory for T cell responses by James Allison and Jeff Bluestone • Ipilimumab (CTLA-4 inhibitor) the first ICI. Trials initiating 2000 • The “Breakthrough” – Allison and Honjo win 2018 Nobel Prize
4. Progress in Immuno-Oncology Did Not Happen Overnight
5. Major Approaches to Immunotherapy • Personalized Cancer Vaccines • CAR-T cells • Immune Checkpoint Inhibition
6. Personalized Cancer Vaccines
7. Personalized Cancer Vaccines First proposed by Paul Ehrlich in 1909 • Somatic mutations can generate cancer-specific neoepitopes that are recognized by autologous T cells as foreign and constitute ideal cancer vaccine targets. • Drivers and passengers • Every tumor has its own unique composition of mutations, with only a small fraction shared between patients. • Technological advances in genomics/proteomics (NGS, mass spectrometry), data science (Bioinformatics), and cancer immunotherapy now enable the rapid mapping of the mutations within a genome, rational selection of vaccine targets, and on-demand production of a therapy customized to a patient’s individual tumor • Both class I MHC (CD8+ CTLs) and class II MHC (CD4+ T helper [TH1] cells) immune responses are involved.
8. Customizing a patient-specific cancer vaccine Ugur Sahin, and Özlem Türeci Science 2018;359:1355-1360 This process can take 3-4 months.
9. Personalized cancer medicine Ugur Sahin, and Özlem Türeci Science 2018;359:1355-1360 The broader impact of realizing personalized mutanome vaccines • Neoepitope vaccines should theoretically be able to target all cancers. • True patient-specific therapy • A delicate interplay between the tumor and the immune system (“cancer-immune set point”) shapes each individual cancer
10. Chimeric Antigen Receptor T Cells (CAR-T cells)
11. Adoptive T cell Transfer Chimeric Antigen Receptor T cells (CAR-T) • autologous T cells engineered to express a chimeric antigen receptor (CAR) specific for the CD19 B lymphocyte molecule have recently been approved by the U.S. Food and Drug Administration ( FDA ) for treatment of refractory pre-B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma • The first successful gene therapy.
12. CAR-T Cell Mechanism of Action June and Sadelain, NEJM, 2018
13. CAR T Cell results June and Sadelain, NEJM, 2018
14. Immune Checkpoint Inhibitors
15. T cells can kill cancer cells • Tumors are immunogenic. Mutations represent “neo-antigens.” • Certain cancers observed to contain TILs, but TILs were dysfunctional • Dysfunction traced to the expression of multiple co-inhibitory receptors that “block” the immune system’s ability to kill the cancer cell – termed “Immune Checkpoints” • Now know that antibody drugs that block these signaling molecules have the ability to release the immune system to kill cancer cells
16. Mutational burden by tumor type Imperfectly predictive of response to immune checkpoint blockade Schumacher, Science, April 2015
17. 41 patients with progresive metastatic carcinoma with or without dMMR/MSI-H
18. PFS and OS of dMMR vs WT on Pembrolizumab Le, NEJM, 2015
19. Based on data from 5 separate clinical trials involving 149 patients who were dMMR/MSI-H. RECIST overall response rate 39.6%. Responses exceeded 6 months in 78% of responders. 11 complete and 48 partial responses. Response rate similar whether the patients were diagnosed with CRC or with other cancers.
20. Timing of clinical development of anti–CTLA-4, anti–PD-1, and anti–PD-L1 antibodies, from first administration to humans to FDA approval Adapted from Antoni Ribas, and Jedd D. Wolchok Science 2018;359:1350-1355 TNBC *This class of agents was the first to be granted FDA approval on the basis of a genetic characteristic as opposed to the site of origin of the cancer, with the approval of pembrolizumab and nivolumab for the treatment of microsatellite-unstable cancers of any origin in 2017
21. Resistance to Immune Checkpoint Inhibition • Success in NSCLC, melanoma, Bladder Ca, RCC, HNSCC, Cervical CA • Many encouraging responses, some apparent cures • But most patients do not respond, and there is the potential of significant side effects => Mechanisms of resistance? => Combination therapy approaches? => Predictive biomarkers?
22. Galluzzi, et al. Science Translational Medicine, 2018
23. Galluzzi, et al. Science Translational Medicine, 2018
24. Galluzzi, et al. Science Translational Medicine, 2018
25. Immune Checkpoint Biomarkers
26. Programmed Cell Death 1 (PD-1) • a dominant negative regulator of antitumor T cell effector function • an “immune checkpoint” • PD-1 has two ligands • PD-L1 (also known as CD274 or B7-H1) • PD-L2 (also known as CD273 or B7-DC) • PD-1 is a negative regulator of preexisting immune responses, which becomes relevant to cancer because its blockade results in preferential stimulation of antitumor T cells
27. Mechanism of action of PD-1–blockade therapy Antoni Ribas, and Jedd D. Wolchok Science 2018;359:1350-1355 T cell activation XT cells excluded from tumor No response T cell activation T cells infiltrate tumor Response
28. The Problem of Resistance Kalbasi, et al, Nat Rev Immunology, 2019
29. Role of IFN signaling • IFN signaling pathway intact => response more likely • IFN signaling altered or diminished => response less likely Keenan, et al, Nat Med, 2019
30. Unmet Medical Needs in the Selection of Immunotherapies - ICIs • Issues with the selection of Immune Checkpoint Inhibitors • Understanding why only small % respond initially => may guide combination IO therapy • Alternative checkpoints engaged? • Loss of HLA expression? • Perturbations in IFN-g signaling? • Understanding why some acquire resistance to ICIs and relapse • Selection for “resistant” clones • Disfavorable microbiome? • Early identification of likely non-responders • ICI therapy costs $150,000.00/year • SAEs are fairly common and sometimes irreversible, or even life-threatening
31. Summary • Multiple types of Immunotherapy • Successful in part, but limited by resistance and toxicity • Enormous potential seems clear • Massive commitment by Pharma to IO drugs and technologies • Complex interactions among tumor cells, immune cells, supporting stroma, gut microbiome, and other host environmental factors offers possibilities for diagnostic illumination • Understanding how various Immune Checkpoints work independently and in concert to evade host immunity is an important next step
About the presenter

Dr. Bates has been developing molecular diagnostics for Oncology applications since 2004, when he headed up Clinical Research for Oncology at Monogram Biosciences/ViroLogic. Dr. Bates has been developing the pipeline of Oncology applications for the GeneXpert at Cepheid since 2011.Dr. Bates trained in Internal Medicine at UCSF, Cardiovascular Diseases at Duke, and Infectious Diseases at the University of Washington.
Related Content
Leica Biosystems content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.