
How to Write a Grant Proposal

Grant writing is a critical part of an investigator’s portfolio. It is a process by which a grant writer requests funding to test/study a novel idea to advance human health.
Funding for research is a highly competitive process. For example, over the last 2-decades, the National Institute of Health (NIH) grant acceptance rate has dipped from 31% in 2002 (30,068 applications / 9,396 awarded) to 19% in 2022 (58,872 applications / 11,229 awarded).1 To increase the odds of funding, a compelling storyline invoking grant makers' immediate attention is necessary.
1. Scope
This document will provide high-level information on the dos and don’ts of grant writing. Essential features increasing the probability of grant award are discussed. This report would be a beneficial read for investigators in the healthcare sector, especially those performing basic and applied research in life science or biomedical sciences.
2. Know the Grant Proposal Guideline
Before putting pen to paper, the investigator needs to inquire if the grantor and the grantee have a common goal. For example, NIH, the largest funding agency in the world, has around 24 institutes and centers that award grant funding. NIH centers include National Cancer Institute, National Heart, Lung, and Blood Institute, National Human Genome Research Institute, etc. Each center has its federal appropriation and specific requisites.
Always keep a regular check on the funding opportunity announcement by the funder. Before initiating the grant proposal, the investigator must thoroughly go through the research grant application to maximize the chances of success. 2
There are several types of research grants that are funded by various agencies. These may include research grants, research career, development grants, small business grants, etc.3 It is necessary for the grantee to perform thorough research on finalizing the type of grant idea pitched to the reviewers. Therefore, visiting the guidelines and requisites of the grant advertisement is highly recommended.
3. Start Early
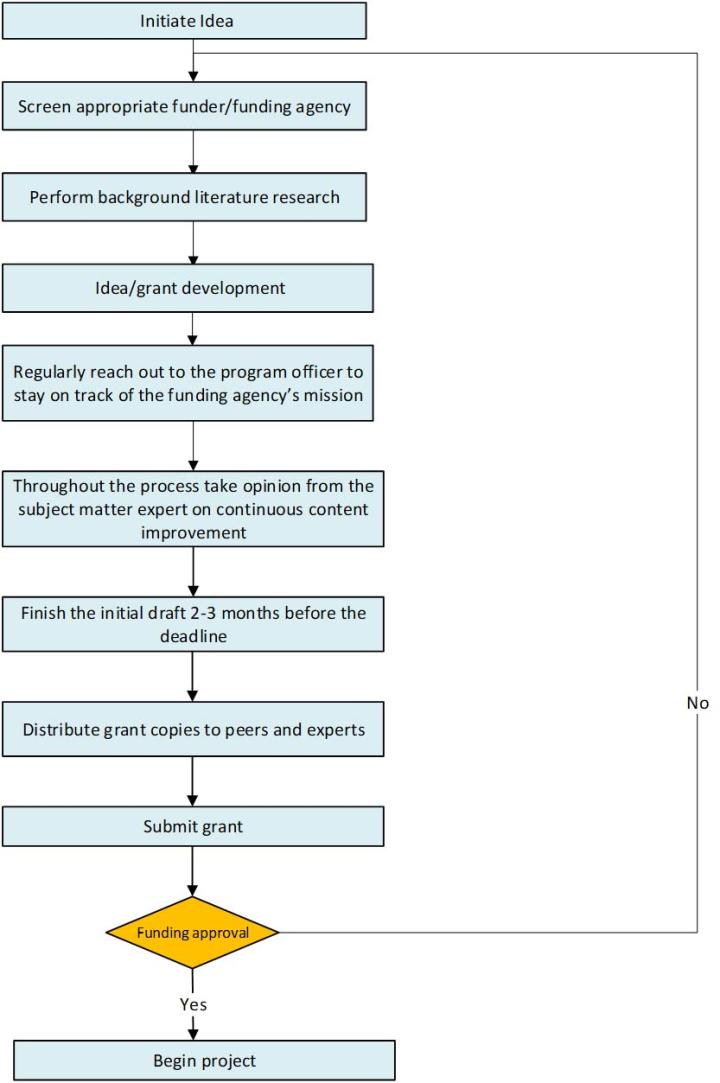
One should start planning for the grant proposal around 6-8 months before submission date. This will give the writer time to discuss the grant content with peers, collaborators, and experts. Starting early will give you a cushion for giving thought to the idea, performing due diligence by sifting through the related literature, and eventually getting a broad understanding of the problem statement.
3.1 Regular connection with the program official
Each grant is assigned to a program officer. A grant program officer can provide insightful feedback to the investigator in the context of the content, the overall flow of the idea, etc. This exercise can give a clear understanding to the investigator of whether the research grant proposal is moving in the right direction. Providing a grant summary to the program officer, including high-level information on the specific aims, can help refine the grant and get continued alignment with the funder’s requirements.
4. Know your Audience
Before initiating the grant writing process, identify the appropriate audience/funding department having a common mission/problem statement. Some tools can help direct the grantee to an appropriate funding agency. For example, NIH’s website uses NIH Reporter that matches the investigator’s proposed abstract to the department most likely to provide the necessary grant aid.4 Once a pertinent institute is identified, a grantee can tailor the relevant information per the funder’s mission statement.
4.1 Succinct biosketch
A biosketch/CV describing an investigator’s qualification and background helps reviewers to better understand the grant writer’s experience and expertise related to the project. Periodically browse the funder's website and format the CV per the latest guidance. A well-crafted biosketch can make a significant impact on the reviewers.
5. Well-Structured Research Grant
A grant should tell a crisp story. Clear emphasis should be provided on the gap in the field and how your effort will close this gap. Finer details must be discussed to the extent that the message should not get redundant or boring. Additionally, from start to finish, one should not lose track of the central hypothesis/primary message.
5.1 Clear aims
Goals and aims should be well-defined. This section is central to your grant.5 The hypothesis-driven aims must be clearly presented. The aims hypothesis should be tested by reliable methods. Always ensure that you design SMART (specific, measurable, actionable, relevant, and time-based) goals. Also, a holistic approach should be presented to the reviewer entailing the scenarios where the hypothesis both succeeds or fails. It is generally recommended that the project aims are independent of each other. This way if the first goal is not accomplished, you can still test your second goal.
Consideration must be given to the fact that the research aims are more important than the broader research area itself. For example, working on cutting-edge research aims is far more important than investigating critical research area such as cancer with redundant specific aims.
5.2 Balanced approach
A grant should have a coherent description to the approach for achieving specific aims. Additionally, details should be provided on the alternate aim in case the primary one is not achieved. Discussing the issues that may arise in the primary goal, corresponding potential pitfalls, and a robust alternate strategy substantially improves the probability of funding for research. The central hypothesis must not be overambitious (especially for early-stage/new investigators). The problem description should include a background section comprising state-of-the-art information.5 The background, with literature-backed evidence, should discuss the central problem and how your research would contribute to the advancement of the field. Also, an extensive literature search will aid in understanding the type and amount of work done in the field. It may also confirm whether your work is novel. If it’s otherwise, you may save time writing the grant further and perhaps go back to the drawing board to re-analyze other important topics.
5.3 Statistical rigor
The supportive data should be backed up by sound statistical inference. Study data can only be conclusive if it undergoes robust statistical analyses, The grant will probably fare poorly if the experiment analysis is not sufficiently powered. For example, if you think that an oncogene is over-expressed in lung cancer tissues, the statement must be buttressed by an appropriate statistical approach.
5.4 Reasonable Budget and Timeline
Provide a clear description of both the direct and indirect project costs. The direct cost would include funding to the scientific staff, reagents, equipment, etc., while the indirect cost may cover institute's sustainability (for example, supporting the administration, upkeep of the building, etc.). The itemized budget must be clearly defined as it highlights the investigator’s vision of successfully running the project. Connect with your institute’s sponsored research office that will assist in developing the budget.3
The grant must also mention the year-by-year timeline of how each specific aim will progress.
6. Rigorous Review of the Content
Proofread the content several times. Ensure that the figure, table, and overall presentation are clear, concise, and devoid of redundancy. If the funding proposal is sloppy with poorly labeled legends, typographical errors, etc., then there are high chances that the reviewer may lose interest and may not even go through the full content.
Provide the draft to a peer/colleague for editing. If necessary, run it through a typing assistant program such as Grammarly. Regularly align with subject-matter experts. This activity will allow you to draft a high-quality impactful grant. For example, if a pathologist is drafting a grant on diagnosing breast cancer using artificial intelligence, then collaborating with an expert computational biologist will improve the chances of funding.
7. Be Persistent
Industry, foundation, and government-funded grants are advertised regularly. Hence, keep looking for these opportunities. In general, most applicants try more than once to get their grant funded. Therefore, if unsuccessful, consistently refine your grant and apply again with a better than before version.
In addition to the NIH, other organizations such as the National Science Foundation, Department of Defense (DoD), Centers for Disease Control and Prevention (CDC), Wellcome trust, World Health Organization, etc., sponsor large and small grants. Also, it always adds value to a grant proposal if you can show funding from other organization(s) as well. Table 1 and Table 2 captures the annual research expenditures by top donors in almost a decade.6,7 A corresponding percent funding breakdown is described in Figure 1 and Figure 2.
Table 1. Top donors in the year 2013
| Organization | Research Expenditure (million $) |
|---|---|
| National Institutes of Health | 26,081.30 |
| European Commission (EC) | 3717.7 |
| UK Medical Research Council (MRC) | 1321.5 |
| Institut national de la santé et de la recherche médicale (Inserm) | 1041.2 |
| United States Department of Defense (US DoD) | 1017.7 |
| Wellcome Trust | 909.1 |
| Canadian Institutes of Health Research (CIHR) | 883.6 |
| Australian National Health and Medical Research Council (NHMRC) | 777.6 |
| Howard Hughes Medical Institute (HHMI) | 752 |
| Deutsche Forschungsgemeinschaft / German Research Foundation (DFG) | 630.6 |
Figure 1. Top donors’ percent funding disbursement for the year 2013
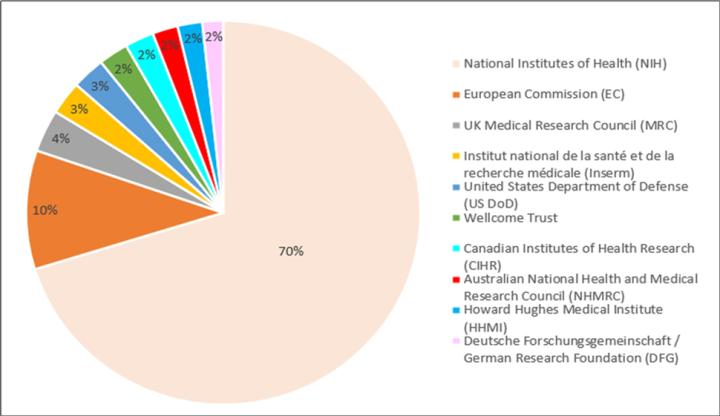
Table 2. Top donors in the year 2019
| Organization | Research Expenditure (million $) |
|---|---|
| National Institutes of Health | 31101.16 |
| Bill & Melinda Gates Foundation | 1083.99 |
| Wellcome trust | 1077.59 |
| UK Medical Research Council | 905.94 |
| Canadian Institutes of Health Research | 832.84 |
| Swedish Research Council | 132.51 |
| European and Developing Countries Clinical Trials Partnership | 120.66 |
| United States Agency for International Development | 64.99 |
| German Federal Ministry of Education and Research (BMBF) | 53.06 |
| Pasteur Network | 6.45 |
Figure 2. Top donors’ percent funding disbursement for the year 2019

8. Discussion
Grant writing may initially look like a cumbersome process; however, if the grantee is aware of the big picture and consistently navigates each section, then it could become a satisfying and rewarding process. The key is to start early, have ample time to perform background research, discuss your ideas with mentor(s) and experts, and then take an initial stab towards grant writing. Take time to write important sections, such as designing your central hypothesis and the corresponding specific aims. Have statistically supportive data to support your claims. The funding probability will increase if this supportive data has prior been published in a peer-reviewed journal.
To make your case stronger, invest time in writing an effective biosketch. Also, reach out to the collaborators and experts to provide strong letters of support. Regularly go through the grantmakers’ guidelines to ensure that the content is according to the funder's needs and requirements. Each section must be written in a manner that should excite the reviewers about the following section. High-quality illustrations and formatting add to easy readability. Figure 3 provides high-level information on the elements needed to increase the likelihood of funding.
Always set realistic expectations of budget and timelines. Do not be overambitious with your grant. Setting up too many specific aims and requesting a hazy budget may not bode well with the reviewers. A simple yet effective idea is generally scored highly by the grantmaker.
9. Conclusion
Grant writing is a long-drawn, complex, and sometimes frustrating process. However, patience, resolve, and passion for a positive change in societal well-being can go a long way in determining an investigator’s success.
About the presenter

Shubham Dayal is a Senior Medical Writer at Leica Biosystems and has over 10 years of experience in regulatory/preclinical/clinical writing for studies that are at different stages of the product lifecycle. Shubham has a PhD in Cell and Molecular Biology from the University of Toledo and a Master's in Regulatory Affairs from Northeastern University and has co-authored multiple peer-reviewed articles and poster presentations. He is an active member of the Regulatory Affairs Professional Society and American Medical Writers Association and holds certifications related to scientific writing. In his current role, Shubham's goal is to create awareness for our customers in ways that can advance scientific communication and ultimately improve patient outcomes.
References
- Research project grants: competing applications,awards, and success rates. Updated February 2022. Accessed June 20, 2022. https://report.nih.gov/nihdatabook/report/20
- Grants & Fundings; NIH central resource for grant and funding information. Updated July 14, 2020. Accessed July 1, 2022. https://grants.nih.gov/grants/how-to-apply-application-guide/format-and-write/write-your-application.htm
- 3Understanding Types of Grants and Funding. Accessed June10, 2022.
https://www.nidcd.nih.gov/funding/types - RePORTER; Matchmaker. Updated July 30, 2022. Accessed August 5, 2022. https://reporter.nih.gov/matchmaker
- Wiseman JT, Alavi K, Milner RJ. Grant writing 101. Clin Colon Rectal Surg. 2013;26(4):228-231. doi:10.1055/s-0033-1356722. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3835460/
- Viergever RF, Hendriks TC. The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds. Health Res Policy Syst. 2016;14:12. Published 2016 Feb 18. doi:10.1186/s12961-015-0074-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4759950/
- World Health Organization: Investments on grants for biomedical research by funder, type of grant, health category and recipient. Updated January 2022. Accessed, August 10,2022. https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/investments-on-grants-for-biomedical-research-by-funder-type-of-grant-health-category-and-recipient
Related Content
Leica Biosystems content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.

