
Optimizing Microtomy, Case Assembly and IHC Testing Through Implementation of Automated Specimen Tracking
The Leica Biosystems Content and Evidence Team has partnered with a private, multi-specialty clinic to assess the impact of implementing automated specimen tracking into their laboratory. This laboratory is most interested in increasing efficiency and patient safety throughout their processes.
During the Optimization Assessment, the Content and Evidence Team observed current processes throughout the histologic process beginning with accessioning and following through to case assembly. As with many laboratories without automated specimen tracking, paper logs or pending reports are used to track samples and users. The staff at this facility currently use LIS generated pending reports to follow cassettes and slides through their processes. Technologists refer to the reports for necessary sample information and to initial the steps that they perform to those samples.
The Leica Biosystems Content and Evidence Team also observed the current processes involved in creating slides. Slide labels are created while grossing dictation is entered into the LIS . The labels are printed from the LIS for each case. The labels are then delivered to the histology area of the laboratory in large groups. The staff spend time placing the labels onto the selected color slides and sorting them by case type and number. When cassettes are ready for microtomy, the technologists search for the matching slides and create a batch. Because there is such a large possibility of matching errors, the laboratory performs a manual block check verification with the slides before sending them to a pathologist.
Through the implementation of an automated specimen tracking system, this laboratory could move towards eliminating the use of the LIS reports and change their labeling and slide creation process to a safer on-demand process. The resulting on-demand slide creation would be verified in the specimen tracking system to ensure that the tissue placed on the slide is from the correct cassette. This would help eliminate the chance of a mis-label during the labeling process in microtomy and reduce the safety risk to the patient. By eliminating these currently required manual steps, the average hands-on time would drop from 171.4 seconds to 147.2 seconds per block in microtomy.
Another area in which a specimen tracking system would increase efficiency is in immunohistochemistry. Since the specimen tracking system could be integrated with their IHC instrumentation, there would no longer be a need for the manual stain ordering and the assignment step. That integration could save more than 29 seconds per slide of hands-on time.
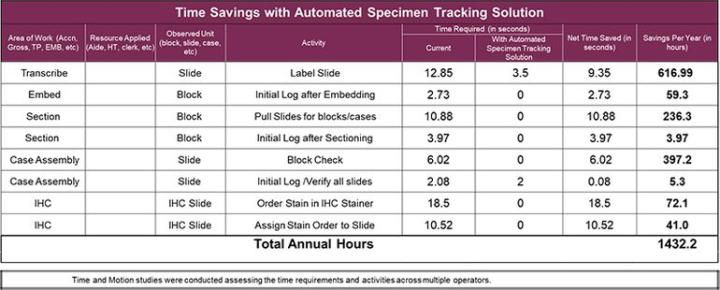
Since the specimen tracking system maintains the necessary information and tracks users throughout the process, the need to refer to or initial logs should be eliminated. The chart below shows specific steps and times savings that this laboratory could realize.
- Elimination of pending log used in 3 areas of the laboratory
- Save approximately 1432 hours/year by eliminating manual tasks
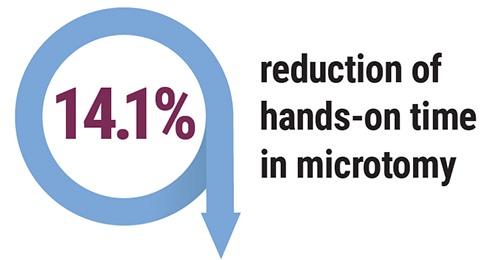
- Reduction of 14.1% hands-on time at microtomy
- Elimination of possible mis-label at grossing and microtomy
- Reduction of 29 seconds per slide in IHC
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
Leica Biosystems content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.