Integrated Solutions. Proven Performance. Reliable Results.
Customer Perspectives

“Thanks to our partnership with Leica, we have access to instruments of the highest caliber. The whole approach is about the quality, traceability, and connectivity… It's a base to work on innovation and development relying on the know-how of the pathologist. We also want to develop other technologies associated with this basis. So, it can be digital pathology technologies based on artificial intelligence.”
Translated from source language.
Dr. Eric Peltier
Président Directeur Général - PRAXEA-DIAGNOSTICS

"Leica Biosystems products are reliable and high quality, and the company is realistic about what's required to transform traditional pathology to a digital, connected practice. I know I can count on them and trust they will be there to support me now and well into the future."
Translated from source language.
Dr. Sylvia L. Asa, MD, PhD
Consultant in Endocrine Pathology, University Hospitals Cleveland Medical Center
News & Promotions
Leica Biosystems Opens New Center for Enabling Precision Medicine to Accelerate Companion Diagnostic Development
Leica Biosystems opened the doors of its new research and development facility in Newcastle Upon Tyne, UK, to accelerate the development of Companion Diagnostics (CDx). CDx tests enable patients to receive targeted therapies to better treat their disease.
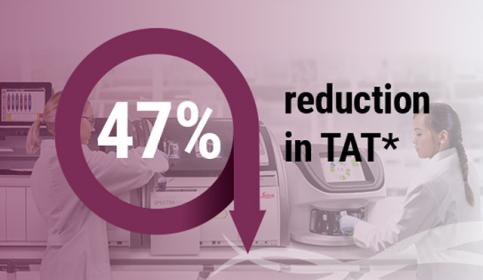
Improved turnaround time and precise digital scanning using glass coverslips
In a recent testimonial, an independent university pathology laboratory compared the slide drying times in a glass coverslipper vs a film coverslipper.
During the demonstration using the HistoCore SPECTRA CV Coverslipper, the customer experienced a 47% reduction in TAT*, reduced exposure to Xylene, and a standardized drying methodology.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
*TAT= turnaround time from coverslipping until slide drying complete