Novel Dual-Stain IHC Assay Enables Discovery with Enhanced Clarity

Contributors
Scott J Anderson, PhD1, Andrew Fuller, MSc1, Stephen Pyper, BSc1, Tom McNicholas, PhD1, Jack Heath, PhD1, Claire Bowen, MD1, Julio Poveda, MD2
1Leica Biosystems, Newcastle Upon Tyne, UK
2University of Miami Miller School of Medicine, Miami, FL
Background
Immunohistochemistry (IHC) analysis has become a powerful and integral component in cancer research. It has been essential in biomarker discovery, with a growing demand for high-output methods that maximize data from limited tissue samples.1,2 ChromoPlex II Dual Detection, an advanced detection product that enables the simultaneous detection of dual stains on a single slide, permits the selection of primary antibodies to be stained in either color, aiding tissue differentiation while minimizing the interference often seen with endogenous pigments or slide debris. The multiplex approach also reduces slide usage compared to single-stains and individual slides. In this study, tissue microarrays with known tumors of distinct tumor histology/classification were utilized to examine differences between the distinct cellular histology (i.e., adenocarcinoma and squamous carcinoma) as well as classical Hodgkin lymphoma (cHL) versus nodular lymphocyte predominant Hodgkin lymphoma (NLP). Another commonly encountered scenario is the difficulty in separating lung adenocarcinoma and bystander macrophages, so these tissues were tested as well. To challenge this staining and imaging workflow, we followed these initial evaluations with quantification of aspects of expression and localization leveraging HALO® image analysis software (Indica Labs).

Methods
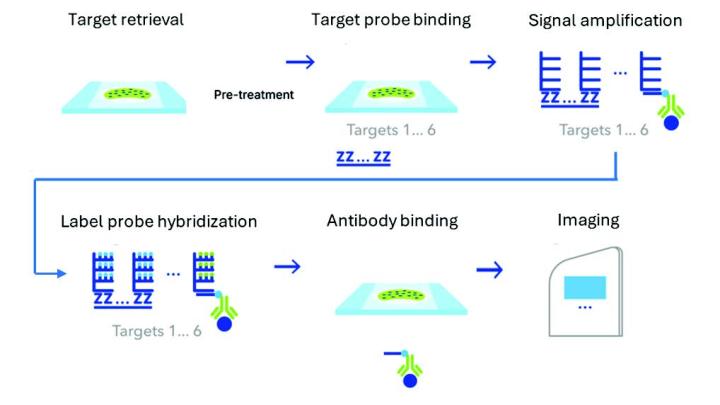
We employed the BOND-III automated platform from Leica Biosystems for the execution of dual IHC assays, enabling precise, reproducible, and highly efficient multiplex staining. Formalin-fixed, paraffin-embedded tissue microarrays were purchased from TissueArray.com. The ChromoPlex II Dual Detection was run using a sequential staining protocol to reduce risk of nonspecific staining and ensure high reproducibility. Each antigen was detected using a distinct chromogen to facilitate clear visualization of multiple markers. The marker combinations and tissues tested are described in the figure legends and were optimized according to pathologist and scientist feedback. Stained slides were scanned using the Aperio GT 450 DX scanner and evaluated by a clinical pathologist for specificity and comparability to single staining. Following this evaluation, HALO image analysis (Indica Labs, research use only) was employed to quantify marker expression and co-localization with the Multiplex IHC module. Image analysis was evaluated by pathologists for accuracy and comparability to qualitative assessment of the slides and images.
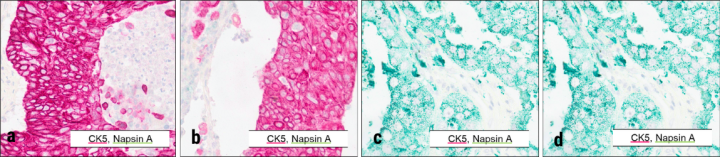
Ready-to-use antibodies from Leica Biosystems were used with the ChromoPlex II Dual Detection immunohistochemistry sequential staining kit. Chromogens indicated in inset legends. Tissue microarrays including squamous carcinoma (a,b) and adenocarcinoma (c,d) were stained using standard optimized protocols on the BOND RX from Leica Biosystems. Imaging (20X) was performed with the Aperio GT 450 DX.
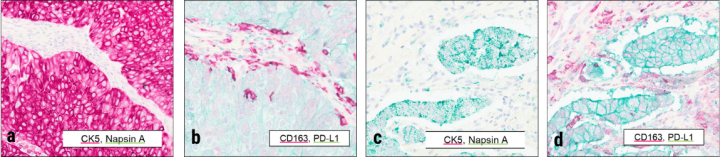
Antibodies against PD-L1 and CD163 from Leica Biosystems were used on the same microarrays described in Figure 2. The ChromoPlex II Dual Detection immunohistochemistry sequential staining kit was deployed with the chromogens indicated in inset legends. Squamous carcinoma (a,b) and adenocarcinoma (c,d) examples are shown comparing both staining protocols in matched regions of tissue. Imaging (20X) was performed with the Aperio GT 450 DX.
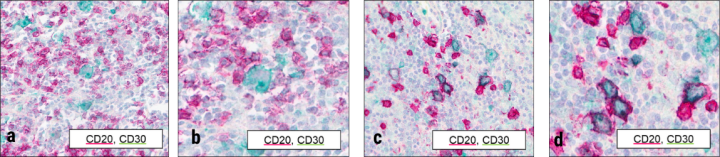
Tissue microarrays for Hodgkin lymphoma were stained with CD20 and CD30 ready-to-use antibodies from Leica Biosystems. Chromogens for each marker are indicated in inset legends. Imaging at 20X (a,c) and 40X (b,d) was performed with the Aperio GT 450 DX to show cellular detail. Upon observation across tissues within the array, pathologists could distinguish CD20-positive and CD30-positive cells in many cases, as shown in the example in (a) and (b). In addition, aberrant co-expression of both markers was identified from this dual staining, as shown in (c) and (d). While this co-expression could be inferred from single staining, the dual stain enabled confirmation of expression of both markers on a subset of cells.
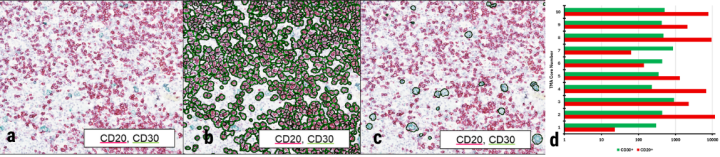
Using HALO software, researchers used the original image shown in (a) to count numbers of CD20- (b) or CD30-positive (c) cells, giving a quick confirmation of qualitative reading of these slides. Statistical analyses showing wide variability in CD20- and CD30-positive cell counts across array cores (d) were performed using the HALO imaging software, reflecting the powerful capabilities of the end-to-end workflow for a myriad of research applications.
Conclusions:
- ChromoPlex II Dual Detection represents a promising tool for high-throughput biomarker studies in research, offering benefits in both clarity of staining and operational efficiency by reducing slide volume.
- Dual staining as shown here can differentiate cell and tissue types and provide spatial information that may be lacking in single IHC staining.
- Multiplex workflow utilizing BOND-III or BOND RX and Aperio GT 450 DX or Aperio GT 450 from Leica Biosystems and HALO image analysis from Indica Labs is a model for discovery and exploration in high-throughput laboratories.
- Additional studies and/or combinations are warranted to explore expanded marker combinations for broader cancer biomarker research applications.
The products shown used in combination are for research use only. Not for use in diagnostic procedures.
About the presenter

Scott Anderson is a Senior R&D leader at Leica Biosystems, bringing over half a decade of expertise in leading research and operational teams. He holds a Ph.D. in Regenerative Medicine and a master’s degree in Immunobiology from the University of Newcastle.
Dr. Anderson is focused on new technologies, such as Artificial Intelligence and Recombinant manufacturing, to support advancements in Leica Biosystems assay development which translate to real life patient benefits.
Scott has co-authored multiple peer review articles and poster presentations and actively supports innovation across Leica Biosystems and its parent company, Danaher.
References
- Harms PW, Frankel TL, Moutafi M, Rao A, Rimm DL, Taube JM, Thomas D, Chan MP, Pantanowitz L. Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists. Mod Pathol. 2023 Jul;36(7):100197. doi: 10.1016/j.modpat.2023.100197. PMID: 37105494. Accessed April 23, 2025 https://www.modernpathology.org/article/S0893-3952(23)00102-3/fulltext
- Poveda J, Cassidy DP, Zhou Y, Chapman J. Easy-To-Perform Dual Immunohistochemistry Can Solve Problems in Hematopathology. Appl Immunohistochem Mol Morphol. 2022 Mar 1;30(3):225-235. doi: 10.1097/PAI.0000000000000991. PMID: 35262526. Accessed April 23, 2025 https://journals.lww.com/appliedimmunohist/abstract/2022/03000/easy_to_perform_dual_immunohistochemistry_can.11.aspx
Related Content
Leica Biosystems content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2026 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.