Fixation and Fixatives (3) – Fixing Agents Other than the Common Aldehydes

As well as formaldehyde and glutaraldehyde, discussed previously, a number of other reagents have been used for fixation, sometimes in simple solution but often combined with other agents in the form of compound fixatives. The more important of these are discussed here in Part 3 of the Fixation and Fixatives series.
Acrolein
Acrolein or acrylic aldehyde (H2C=CH.CHO) reacts with macromolecules in a similar fashion to formaldehyde forming reversible cross-links. It has been shown to produce more cross-links than formaldehyde. Acrolein reacts with fatty acids through its double bond. Although acrolein penetrates tissue rapidly and is extremely reactive it has not found widespread acceptance because it is unpleasant to use (a powerful lacrimatory agent), unstable at alkaline pH, and readily forms polymers. It has mainly been used for enzyme histochemistry and for the fixation of plant material. Following fixation residual carbonyl groups in the tissue will cause background staining with techniques such as PAS. 1-3
Glyoxal
Glyoxal or diformyl (CHO.CHO) is a bifunctional aldehyde that reacts in a similar manner to formaldehyde producing a similar morphological picture. Each of the aldehyde groups is potentially reactive and cross-links can be formed. Glyoxal is the major ingredient in several proprietary fixatives particularly applicable to microwave fixation as well as for routine work. It is commonly termed a “formalin substitute” as it is claimed to be less hazardous in use than formaldehyde because it produces virtually no vapors at room temperature having an extremely low vapor pressure. Although it is an irritant and potentially sensitizing on skin contact it is not currently classified as a carcinogen, although warnings are provided as to its potential mutagenic effects. It reacts with DNA forming adducts in an unstable and reversible manner. It is said to be biodegradable so that disposal will be easier than for formalin. 2, 4, 5
Osmium tetroxide
Osmium tetroxide (OsO4) is a highly toxic, volatile, crystalline solid which is supplied in sealed ampoules. Because of its volatility it must be handled with great care under a fume hood because it will readily fix the conjunctiva of the eye and the nasal mucosa.6 It is soluble in polar and non-polar solvents and reacts with the side chains of proteins forming cross-links. The most important fixation reactions of osmium tetroxide are those involving unsaturated bonds of lipids and phospholipids as it is one of the few fixatives that stabilizes lipids. During fixation osmium tetroxide is reduced to lower oxides which are black and insoluble and these are deposited in tissues, particularly on membranes. Because osmium is a heavy metal it scatters electrons and thus adds electron density to the electron microscope image.7, 8 It can also be used as an en bloc stain for demonstrating lipids (particularly myelinated nerve fibers) at the light microscope level. Generally it is used at about 1% w/v as a specialized secondary fixative for electron microscopy and for teased nerve fibre preparations.9
Carbodiimides:
Carbodiimides (RN=C=NR’) are very reactive compounds that will react with a range of functional groups. The reactions can be enhanced or selectively blocked by choosing a particular type of carbodiimide, pH, temperature, or catalyst. Because peptide bonds may form as one result of the fixation reactions and subsequently these may be selectively broken using proteases, this group of compounds is thought to have some potential for use in immunohistochemistry as well as for routine histology. Carbodiimides are already used for the preparation of immunogens.2
Other cross-linking agents
Diimidoesters are water-soluble compounds which cross-link amino groups of proteins and have been used for electron microscopy and immunohistochemistry. Chloro-s-triazides (cyanuric chloride) has been used for salivary gland mucins and immunofluorescence. Diisocyanates have been used to attach fluorescent tags to proteins, while Diethylpyrocarbonate (DPC) reacts with tryptophan residues and has been used as a vapour-phase fixative for freeze-dried tissue. In an appropriate buffer solution it has been proposed as a fixative for small specimens. Maleimides appear to form some cross-links with proteins and Benzoquinone reacts with amines, amino acids and peptides and has been used to fix peptides in endocrine tissues for immunohistochemistr.2, 3
Mercuric chloride
Mercuric chloride (HgCl2) was one of the first reagents used for tissue fixation. In older literature it was referred to as “corrosive sublimate”, an apt name because it is a corrosive chemical which reacts with metals including the stainless steel of dissecting instruments. Although the mechanisms by which it fixes tissue are not fully understood it is known to react with amines, amides, amino acids and sulphydryl groups, the latter being prominent in its reaction with cysteine where it is thought to produce cross-links. It is a powerful protein coagulant which leaves tissue in a state which produces strong staining with acid dyes. It reacts with phosphate residues of nucleic acids and effectively fixes nucleoproteins. It is for this reason that it is the major component in formulated fixatives such as B-5 and Helly’s, fixatives recommended when high quality nuclear preservation is required (e.g. bone marrow trephines).
There are several disadvantages to using fixatives containing mercuric chloride. Apart from the corrosive nature of mercuric chloride, mercury is highly toxic, can be absorbed through the skin and is a cumulative poison. In most countries there are strict rules about disposal of mercury and compounds containing mercury. During fixation with fixatives containing mercuric chloride a crystalline or amorphous greenish-brown artefact pigment of mercury is randomly deposited in tissues. Treatment of specimens with iodine (Lugol’s iodine) during processing or sections prior to staining, will produce mercuric iodide which can be washed out of the tissues. A subsequent treatment with sodium thiosulphate then removes residual iodine. Mercuric chloride-based fixatives tend to penetrate poorly and if fixation is prolonged tissues become very hard and are prone to shrinkage during processing.6, 7
In recent years a number of metal salts have been introduced as substitutes for mercuric chloride including salts of zinc and barium. Zinc chloride and zinc sulphate have been accepted fairly widely as being suitable and there are now many proprietary B-5 substitutes available.6
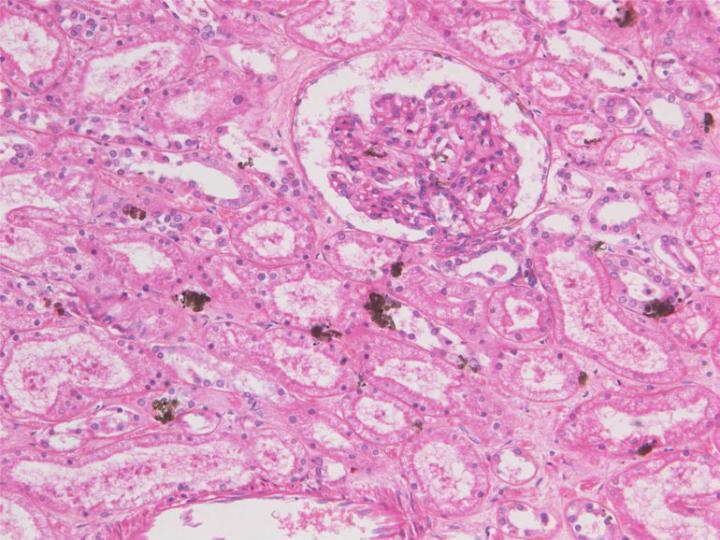
Zinc salts
Zinc sulphate (ZnSO4) and zinc chloride (ZnCl2) are used as substitutes for mercuric chloride in a number of formulated and proprietary fixatives, the sulphate being more popular because it is potentially less corrosive than the chloride which has been reported as causing problems in tissue processors6 (see Part 4). The salts are added to 10% formalin solutions (aqueous or alcoholic, buffered or un-buffered), at a concentration of about 1%, but there have been some problems reported with precipitation of zinc or buffer salts during preparation. Zinc salts will react with a range of tissue end groups including amino, carboxyl and sulphydryl, forming reversible reaction products some of which can be removed with a citrate or EDTA wash. Zinc is said to enhance fixation and staining, particularly of nuclei, in a similar way to mercuric chloride. It is claimed to have advantages in preserving immuno-reactivity when compared to formalin alone obviating the need for antigen retrieval for some epitopes. Zinc salts are far less toxic than mercury salts and disposal of zinc solutions should pose no problem 2, 6.
Picric acid
Picric acid or trinitro phenol (C6H2(NO2)3OH) is a bright yellow crystalline substance that must be stored wet with water to avoid the risk of explosion by percussion or heating of the dry substance. It should be kept in a tightly sealed container and regularly checked to see that it is damp.10 Distilled water can be added if it appears to be drying out. For fixation it is always used in combination with other agents (see Part 4 – Bouin’s and Hollande’s solutions). Apart from being a component of fixatives picric acid is used as an acid dye in several stains (eg. in Van Gieson’s solution for staining muscle). It imparts a yellow color to tissues during fixation and because of its acidic nature residual picric acid should be washed from tissues with 70% ethanol before processing. If residual picric acid remains in tissue blocks after processing the staining characteristics of the tissue will be affected and will deteriorate in time.
Picric acid is a coagulant fixative which changes the charges on the ionisable side chains of proteins and disrupts electrostatic and hydrogen bonds. It forms salts (picrates) with basic groups of proteins causing coagulation. It appears not to fix lipids or most carbohydrates but is recommended as a component in fixatives used to preserve glycogen. Picric acid can hydrolyse nucleic acids, so it should be avoided if DNA or RNA are to be demonstrated. Picric acid will dissolve small deposits of calcium in specimens. A considerable amount of shrinkage occurs during the processing of tissues fixed in picric acid containing reagents.6, 7, 11
Potassium dichromate
Potassium dichromate (K2Cr2O7) behaves as a non-coagulant unless it is used at pH < 3.4 – 3.8, where it reacts like chromic acid, as a coagulant. It is a component of several compound fixatives (see Part 4 – Zenker’s and Helly’s solutions). The fixation reactions are thought to involve the oxidation of proteins with the interaction of reduced chromate ions forming some cross-links, the extent being determined by the pH of the fixative. Chromium ions are reported to react with carboxyl and hydroxyl side chains of proteins. It leaves amino groups available and thus favours staining with acid dyes. Chromate will react with unsaturated lipids rendering them insoluble, it being for this reason that it is regarded as a good fixative for mitochondria. It is normally recommended that tissues fixed in a chromate containing fixative are thoroughly washed in water prior to processing to avoid the formation of an insoluble chromate sub-oxide by reaction of processing alcohol and the chromate salt. Traditionally dichromate containing fixatives were used in histochemical methods for the amine containing “chromaffin” granules of endocrine tissues.6, 7, 11
Ethanol and methanol
Ethanol (CH3CH2OH) and methanol (CH3OH) are considered to be coagulants that denature proteins. They replace water in the tissue environment disrupting hydrophobic and hydrogen bonding thus exposing the internal hydrophobic groups of proteins and altering their tertiary structure and their solubility in water. Methanol is closer to the structure of water than ethanol so ethanol interacts more strongly with hydrophobic areas than methanol. Fixation commences at a concentration of 50 – 60% for ethanol and >80% for methanol. Ethanol is sometimes used to preserve glycogen but will cause distortion of nuclear and cytoplasmic detail. Methanol is commonly used as a fixative for blood films and 95% ethanol is used as a fixative for cytology smears but both alcohols are usually combined with other reagents when used as fixatives for tissue specimens.3, 7, 12
Acetone
Acetone (CH3COCH3) has a similar action to alcohol and has been used as a fixative and dehydrant for tissue processing, particularly rapid hand-processing of small specimens. It is widely recommended for fixation as part of the histochemical demonstration of enzymes where it is generally used cold (4°C). It is an effective lipid solvent with a rapid action which can make tissues very brittle. Because it is highly volatile and flammable it is generally not used on automatic tissue processors.3, 7
Acetone should not be used on some tissue processors because it will adversely affect seals and other components of the equipment.
Acetic acid
Acetic acid (CH3COOH) is coagulant in action with nucleic acids but generally does not fix proteins. It is incorporated in compound fixatives to help prevent the loss of nucleic acids and, because it swells collagen, to counter the shrinkage caused by other ingredients such as ethanol. Acetic acid penetrates very rapidly but fixatives that contain it will lyse red blood cells. 3, 7
About the presenter

Geoffrey Rolls is a Histology Consultant with decades of experience in the field. He is a former Senior Lecturer in histopathology in the Department of Laboratory Medicine, RMIT University in Melbourne, Australia.
References
- Culling CFA, Allison RT, Barr WT. Cellular pathology technique. London: Butterworths, 1985;32.
- Eltoum I, Fredenburgh J, Grizzle WE. Advanced concepts in fixation: 1. Effects of fixation on immunohistochemistry, reversibility of fixation and recovery of proteins, nucleic acids, and other molecules from fixed and processed tissues. 2. Developmental methods of fixation. J Histotechnol 2001;24;201-210.
- Leong AS-Y. Fixation and fixatives. In Woods AE and Ellis RC eds. Laboratory histopathology. New York: Churchill Livingstone, 1994;4.1-1 - 4.1-26.
- Fisher Scientific. MSDS :Glyoxal. 2008; . http://fscimage.fishersci.com/msds/01960.htm; November 22, 2011
- World Health Organization. Concise International Chemical Assessment Document 57 GLYOXAL. 2004; http://whqlibdoc.who.int/publications/2004/924153057X.pdf; November 22
- Carson FL. Histotechnology. 2nd ed. Chicago: ASCP Press, 2007.
- Eltoum I, Fredenburgh J, Myers RB, Grizzle WE. Introduction to the theory and practice of fixation of tissues. J Histotechnol 2001;24;173 -190.
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. Boston: Jones and Bartlett, 1992.
- Lowe J. Techniques in neuropathology. In Bancroft JD and Stevens A eds. Theory and practice of histological techniques. New York: Churchill Livingstone, 1996;373.
- Sigma-Aldrich. MSDS Picric acid solution. 2011; http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en&N4=197378|ALDRICH&N5=SEARCH_CONCAT_PNO|BRAND_KEY&F=SPEC; November 22
- Drury RAB, Wallington EA. Carleton's histological technique. . 5th ed. New York: Churchill Livingstone, 1980.
- Lillie RD, Fullmer HM. Histopathologic technique and practical histochemistry. 4th ed. New York: McGraw-Hill, 1976;45.
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.