
Factor XIIIa Expression in Normal and Pathological Skin


FXIIIa is a blood and intracellularly produced coagulation factor, which is widely expressed in a variety of cell types. It is believed to be vital in the final stages of the clotting cascade and is also important in wound healing and repair mechanisms. It also has a role in angiogenesis in embryo implantation. In normal skin, it is widely expressed in cells termed dermal dendrocytes, which are derived from a monocyte/macrophage or fibroblastic cell lineage. The cells are located predominantly in the papillary dermis at the dermo-epidermal junction, also in association with skin appendages, but normally not within the epidermis. Elevated numbers of FXIIIa positive cells can be found in a host of cutaneous inflammatory conditions, including lichen planus and psoriasis. In tumor pathology, its main application is to help distinguish immunophenotypic differences between dermatofibromas (DF) and a host of other fibrohistiocytic entities, most notably dermatofibrosarcoma protuberans (DFSP).
Introduction to Factor XIIIa
Factor XIIIa is a blood coagulation prototransglutaminase1, which becomes an activated component of the final stages of the clotting cascade as a result of the interaction with thrombin and Ca2+. FXIIIa plays a key role in fibrin stabilization, and defective fibrin stabilization is demonstrable as a result of severe haemorrhagic manifestations of congenital FXIII deficiency, which is an autosomal recessive disorder. In the plasma form, the enzyme is a heterotetramer composed of paired A and B subunits (A2B2). In the tissue or cellular form, the protein lacks the B subunits and is therefore structurally a homodimer of A subunits (A2). Genetic studies have mapped the gene coding for the A and B subunits to chromosomes 6p24–25 and 1q31–32.1, respectively. Studies on placental tissue using cDNA coding for human factor XIIIa revealed isolated factor XIIIa cDNA size to be composed of 3905 bases, which code for a protein composed of 732 amino acids.2 Cells of the bone marrow origin are believed to be the primary site for the synthesis of the subunit A in plasma FXIII, hepatocytes in the liver have also been implicated. The subunit B of plasma FXIII is synthesized in the liver.
As well as a functional homeostatic role in clot formation, FXIII has a significant role in wound healing3 and the embryo implantation processes4, which involve angiogenic pathways5. In the case of wound healing FXIIIa is believed to be central to reducing vascular endothelial permeability during the repair process and is a potential new strategy in the treatment of increased vascular permeability particularly in diabetic wounds arising due to chronic venous insufficiency. In terms of embryo implantation FXIII is believed to have pro-angiogenic properties, which are essential in maintaining healthy pregnancies.
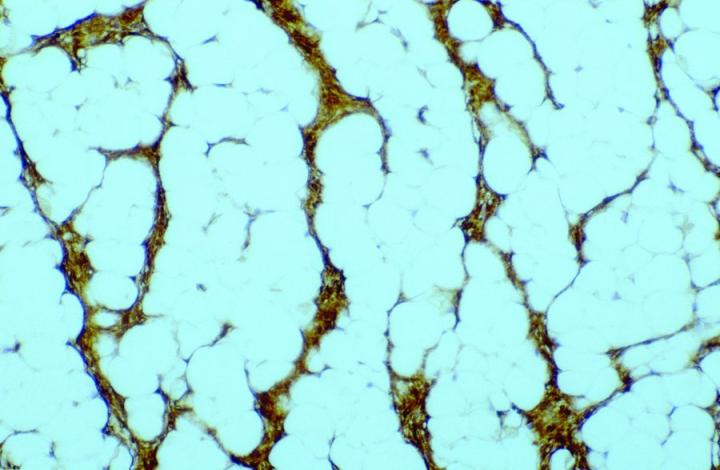
Antibody demonstration of FXIIIa in tissue sections has been available since the late 1980’s, with seminal publications form Cerio and Headington.6 Its distribution in normal tissue is widespread, a publication by Derrick et al7 found FXIIIa positive cells in all organ types assessed including skin, esophagus, stomach, small bowel, large bowel, bladder, lung, kidney, liver, spleen, testis, and thyroid. In the authors' experience, FXIIIa positive cells can be seen in a much wider variety of tissue types, particularly in those with any encapsulated lymphoid structures, notably in connective tissue surrounding, or penetrating into such tissues, in particular, sinus lining cells. High levels can be seen in septa between fat lobules. However, in normal states, elevated levels of FXIIIa cells could be seen in skin and mucosal tissue (gastrointestinal tract and bladder), with such sites as liver, thyroid, testis and spleen only demonstrating relatively low numbers. The significant rationale is that the elevated numbers of FXIIIa cells in such tissues suggests that these cells have an important role in immune responses. In the skin, the distribution of positive cells is confined to the dermis, with the significant numbers being recorded within the papillary dermis (Figure 1), at the dermo-epidermal junction, in loose areolar connective tissue around appendages, particularly pilosebaceous units and around vessels.8
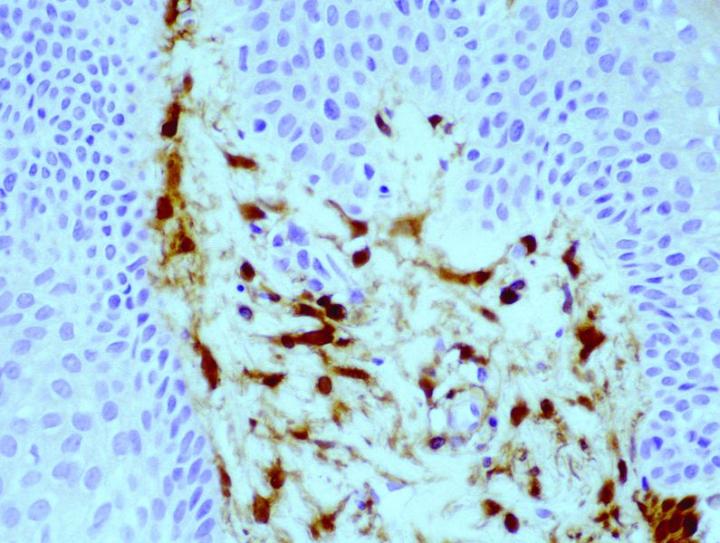
The antigenic epitope appears to be localized predominantly to the cytoplasm of the cells, with some membraneous labelling reported.8 The FXIIIa cells appear to have differing structural appearances depending on the location of the cells. In the dermo-epidermal junction of the skin, they appear as large demarcated dendritic cells, with prominent cell bodies and tapering cell processes, most of which are orientated towards the epidermis. Conversely, the cells located deeper within the dermis are narrow and more clumped in numbers, and the dendritic processes are less pronounced. The striking appearance of these cells leads to the term "dermal dendrocyte" as a descriptive identification of these cells, first introduced by Headington.9
These cells of bone marrow origin have particular histoenzymatic and immunohistochemical features, which have similarities to other cells exhibiting antigen-presenting cell features, however, they are quite distinct from Langerhans cells. They in fact do not express CD1a and unlike Langerhans cells have been shown to be phagocytic.6 Preliminary studies indicated that the dermal dendrocyte was not derived from a fibroblast lineage.6 & 8 Moreover negative labelling for FXIIIa in fibrocollagenous cutaneous disorders reaffirmed this earlier viewpoint.8 Conversely, Nemeth et al10 have reported FXIIIa positivity in dendritic fibroblasts in fibrous papules of the nose, acquired digital fibrokeratomas, angiofibromas and oral fibromas. Observations from this study indicate that FXIIIa may be acting as a growth factor in these tumors or that these tumors are arising as a consequence of overproduction of FXIIIa, in response to an undefined stimulus. In addition, in the authors' experience, there is also the observation that elevated numbers of FXIIIa positive cells are often found at the base or surrounding any invasive cutaneous tumor, and that such cells are often negative for conventional macrophage markers such as CD68.
These FXIIIa positive cells do share certain cell surface markers with cells of the monocyte/ macrophage lineage notably (OKM5+, Mo1+, Mono1+, and Leu M3+). Recent studies on FXIIIa gene expression and protein production in long term cultures of human monocytes, inducing either classical or alternative activation pathways and employing quantitative RT-PCR and fluorescent image analysis at the single-cell level, reveals that the expression of FXIIIa at both the mRNA and protein level is inversely regulated during the two activation routes. Torocsik et al11 concluded that FXIIIa expression is a marker for alternatively activated macrophages, while the absence of FXIIIa in monocyte-derived macrophages is an indicator of their activated state.
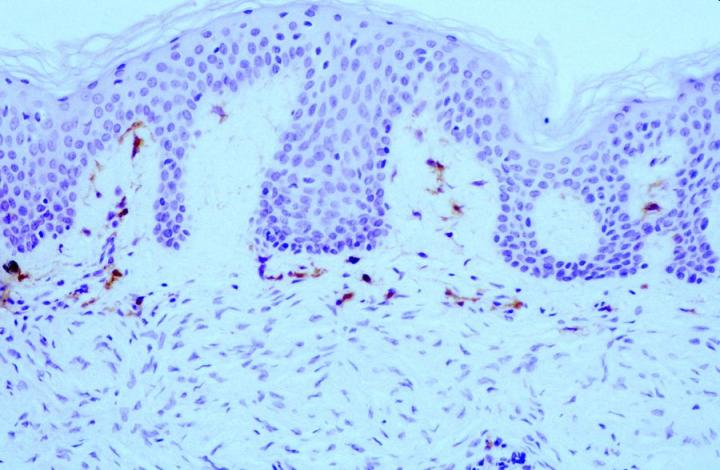
Broadly speaking, FXIIIa positive cells in pathological skin conditions can be split into expression in inflammatory conditions and tumor pathology. In terms of inflammatory states FXIIIa expression is elevated in conditions such as spongiotic dermatiti and psoriasis.6 & 8 In both acute and chronic graft-versus-host disease (GVHD), expression of FXIIIa positive cells is elevated12, with one report indicating a difference between the acute (decreased) and the chronic form (increased).13 In most conditions, it is also the case that elevated numbers of Langerhans cells (CD1a) positive are also detected. Migration of FXIIIa positive cells into the epidermal compartment has been reported in cases of patients with chronic plaque psoriasis in association with other inflammatory cells8, again supporting the notion that these cells have an APC role within the skin.
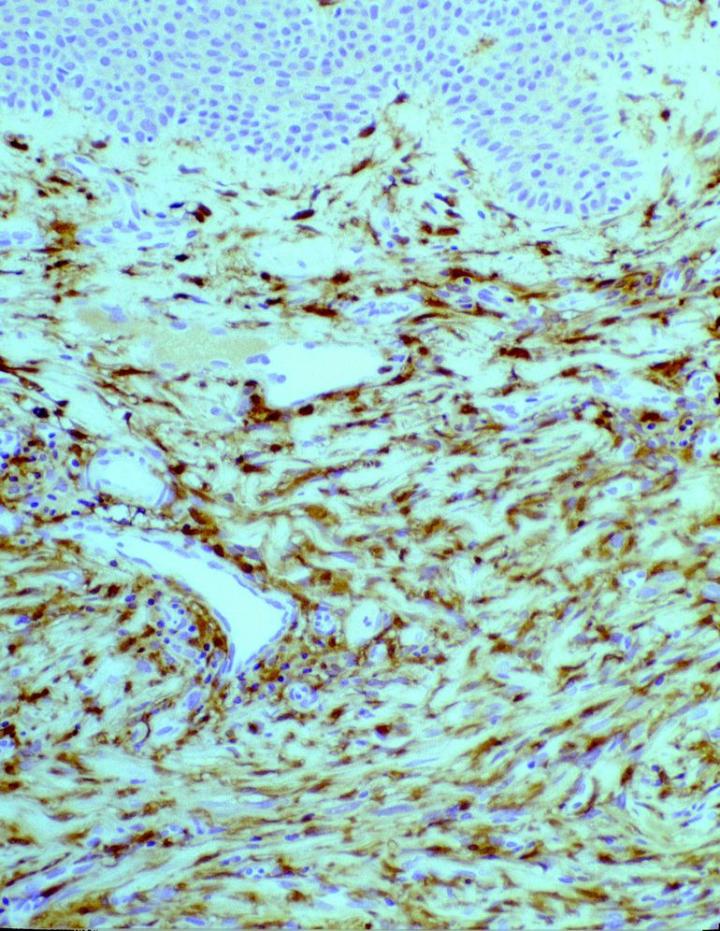
In tumor pathology, FXIIIa has found a value in certain cutaneous mesenchymal tumors. Early reports published by Jones et al14 and Cerio et al15 demonstrated that FXIIIa was elevated in epithelioid cell histiocytoma and dermatofibromas (Figures 2 and 3).
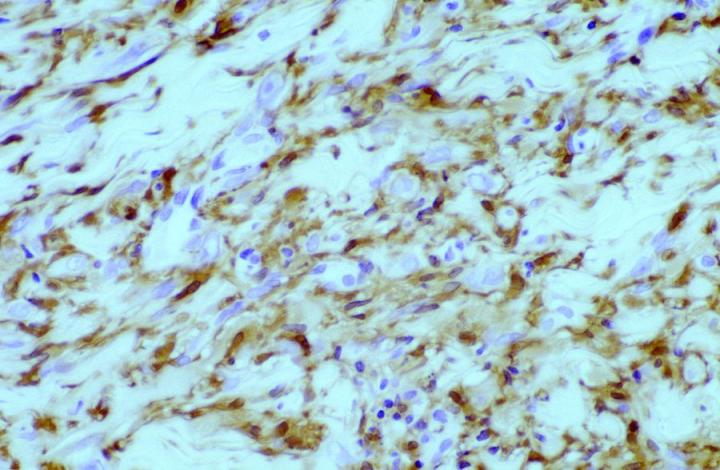
Approximately 50% of the constituent tumor cell population were reported positive in both papers. Labeling was noticed to be weaker towards the center of the lesions. This observation is very useful in the context of distinguishing, dermatofibromas (DF) from dermatofibrosarcoma protuberans (DFSP). The variation in histological appearances of DF and DFSP is extensive, and the differential diagnosis can be difficult. However, immunophenotypically DFSPÕs are CD34 positive (Figures 4 & 5) whereas dermatofibromas express FXIIIa.
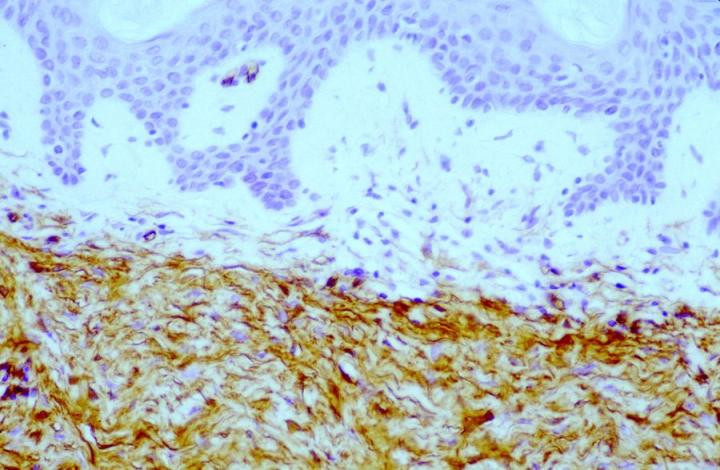
Patchy FXIIIa labelling within DFSP can be seen, but in most cases, positive cells are, in fact, entrapped non-neoplastic dermal dendrocytes or surrounding the periphery of the invasive tumor cells (Figure 6).16 In the authors’ experience, it is also possible to see focal labelling with CD34 in DF in some cases, the significance of which remains unclear.
Immunoelectronmicroscopic examination of FXIIIa-positive cells in DF reveal that these cells resemble dermal dendrocytes in that they display moderate to abundant rough endoplasmic reticulum, lipid droplets and/or bundles or myofilaments in varying proportions.17 There is also evidence of macular adherence connections between neighboring cells. Studies on malignant fibrous histiocytomas (MFH), reveal FXIIIa expression in cells to a variable degree. It is felt that since these tumors have a wide spectrum of histological appearances, all of which are a variation of spindled fibroblast-like cells, undifferentiated cells and histiocytic or histocyte like cells, the FXIIIa positive cells represent different levels of fibrohistiocytic differentiation and are in fact fibrohistiocytic precursors.18
FXIIIa is also widely expressed in several tumors belonging to the series of non-Langerhans cell histiocytoses, such as xanthoma disseminatum, and xanthogranulomas.19, 20
FXIIIa has also been reported to be positive in cellular neurothekeomas21, a relatively rare benign cutaneous neoplasm occurring commonly in young adults on the head and neck area. FXIIIa positive cells have also been reported in granular cell tumors of the skin.22 Most granular cell tumors of the skin are derived from Schwann cell origin, based on immunohistochemical and electron microscopic studies. The presence of FXIIIa-positive cells in granular cell tumors of the skin indicates that these tumors may be an exception in the group of Schwannomas.
The expression of FXIIIa in spindle-shaped cells in Kaposi sarcoma may be of some significance. Kaposi's sarcoma is a neoplasm that is often multifocal and involves multiple organ involvement but quite commonly affects the skin. It may be that the presence of dermal dendrocytes is important to the angioproliferative response in this particular condition.23
About the presenters

Guy Orchard is a Consultant Grade Biomedical Scientist and Laboratory Manager at Viapath, St John's Institute of Dermatology, London. He has been a council member for the Institute of Biomedical Science (IBMS) and is currently a specialist advisor for cellular pathology and the Deputy Chief Examiner for Cellular Pathology for the IBMS. In 2015, Guy was awarded the Leica Leadership Award for Teaching by the National Society for Histotechnologists.”

Catherine Stefanato is a Dermatopathology Consultant in the Department of Dermatology at St. John's Institute of Dermatology, London, UK. Dr. Stefanato completed her residency training in Dermatology at the Catholic University of the Sacred Heart in Rome, Italy. She undertook residency training in Anatomic Pathology at Yale University School of Medicine (USA), followed by Fellowship training in Dermatology at the Boston University School of Medicine, where she also worked as a Consultant Dermatopathologist and Assistant Professor of Dermatology and Pathology. Dr. Stefanato is a Diplomate of the American Boards of both Anatomic Pathology and Dermatology.
Referenzen
- Muszbek l, Adany R, Mikkola H. Novel aspects of blood coagulation factor XIII. I. Structure, distribution, activation, and function. Crit Rev Clin Lab Sci 1996; 33 (5): 357–421.
- Grundmann U, Amann E, Zettlmeissi G, Kupper HA. Characterization of cDNA coding for human factor XIIIa. Proc Natl Acad Sci USA 1986; 83 (21): 8024–8.
- Wozniak G, Noll T. Factor XIII and wound healing. Hamostaseologie 2002; 22 (1): 59–62.
- Tosetto A, Castaman G, Rodeghiero F. Acquired plasma factor XIII deficiencies. Haematologica 1993; 78 (6 Suppl): 5–10.
- Inbal A, Dardik R. Role of coagulation factor XIII (FXIII) in angiogenesis and tissue repair. Pathophysiol Haemost Thromb 2006; 35 (1–2): 162–5.
- Cerio R, Griffiths CE, Cooper KD, Nickoloff BJ, Headington JT. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol 1989; 121 (4): 421–31.
- Derrick EK, Barker JN, Khan A, Price ML, Macdonald DM. The tissue distribution of factor XIIIa positive cells. Histopathology. 1993; 22 (2): 157–162.
- Cerio R, Spaull J, Oliver GF, Jones WE. A study of factor XIIIa and MAC 387 immunolabeling in normal and pathological skin. Am J Dermatopathol 1990; 12 (3): 221–233.
- Headington JT. The dermal dendrocyte. Adv Dermatol 1986; 1: 159–171.
- Nemeth AJ, Penneys NS. Factor XIIIa is expressed by fibroblasts in fibrovascular tumors. J Cutan Pathol 1989; 16 (5): 266–71.
- Torocsik D, Bardos H, Nagy L, Adany R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol Life Sci 2005; 62 (18): 2132–9.
- Yoo YH, Park BS, Whitaker-Menezes D, Korngold R, Murphy GF. Dermal dendrocytes participate in the cellular pathology of experimental acute graft-versus-host disease. J Cutan Pathol 1998; 25 (8): 426–34.
- Deguchi M, Aiba S, Ohtani H, Nagura H, Tagami H. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft- versus_host disease. Arch Dermatol Res 2002;294(7):297-302.
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol 1989; 120 (2): 185–95.
- Cerio R, Spaull J, Jones EW. Histiocytoma cutis: a tumour of dermal dendrocytes (dermal dendrocytoma). Br J Dermatol 1989; 120 (2): 197–206.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathol 1997; 19 (2): 147–53.
- Song Y, Sakamoto F, Ito M. Characterization of factor XIIIa+ dendritic cells in dermatofibroma: Immunohistochemical, electron and immunoelectron microscopical observations. J Dermatol Sci 2005; 39 (2): 89–96.
- Nemes Z, Thomaszy V. Factor XIIIa and the classic histiocytic markers in malignant fibrous histiocytoma: a comparative immunohistochemical study. Hum Pathol 1988; 19 (7): 822–9.
- Zelger B, Cerio R, Orchard G, Fritsch P, Wilson-Jones E. Histologic and immunohistochemical study comparing xanthoma disseminatum and histiocytosis X. Arch Dermatol 1992; 128 (9): 1207–12.
- Zelger BG, Orchard G, Rudolph P, Zelger B. Scalloped cell xanthogranuloma. Histopathology 1998; 32 (4): 368–74.
- Mahalingam M, Alter JN, Bhawan J. Multiple cellular neurothekeomas – a case report and review on the role of immunohistochemistry as a histologic adjunct. J Cutan Pathol 2006; 33 (1): 51–6.
- Nikkels AF, Arrese Estrada J, Pierard-Franchimont C, Pierard GE. CD68 and factor XIIIa expressions in granular-cell tumor of the skin. Dermatology 1993; 186 (2): 106–8.
- Nickoloff BJ, Griffiths CE. The spindle-shaped cells in cutaneous Kaposi’s sarcoma. Histologic simulators include factor XIIIa dermal dendrocytes. Am J Pathol 1989; 135 (5): 793–800.
Related Content
Die Inhalte des Knowledge Pathway von Leica Biosystems unterliegen den Nutzungsbedingungen der Website von Leica Biosystems, die hier eingesehen werden können: Rechtlicher Hinweis. Der Inhalt, einschließlich der Webinare, Schulungspräsentationen und ähnlicher Materialien, soll allgemeine Informationen zu bestimmten Themen liefern, die für medizinische Fachkräfte von Interesse sind. Er soll explizit nicht der medizinischen, behördlichen oder rechtlichen Beratung dienen und kann diese auch nicht ersetzen. Die Ansichten und Meinungen, die in Inhalten Dritter zum Ausdruck gebracht werden, spiegeln die persönlichen Auffassungen der Sprecher/Autoren wider und decken sich nicht notwendigerweise mit denen von Leica Biosystems, seinen Mitarbeitern oder Vertretern. Jegliche in den Inhalten enthaltene Links, die auf Quellen oder Inhalte Dritter verweisen, werden lediglich aus Gründen Ihrer Annehmlichkeit zur Verfügung gestellt.
Vor dem Gebrauch sollten die Produktinformationen, Beilagen und Bedienungsanleitungen der jeweiligen Medikamente und Geräte konsultiert werden.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.



