
DER VORTEIL MEHRERER DURCHLÄUFE ZUR GEWEBEINFILTRATION

Die Gewebeinfiltration ist ein entscheidender Schritt im Histologieprozess, um zeitnah hochwertige Ergebnisse zu erhalten. Dabei werden Gewebeproben mit Paraffinwachs infiltriert und so in einen Zustand versetzt, in dem sie sich in dünne Scheiben schneiden lassen und schließlich gefärbt werden können.'Fixiermittel, Verarbeitungsprotokolle, Gewebetyp und Probengröße sind bei der Gewebeinfiltration unbedingt zu berücksichtigen, um optimale Resultate zu erreichen.1 Freida Carson zufolge besteht die größte Herausforderung in der Gewebeinfiltration darin, Biopsien und größere (Routine-)proben gleichzeitig im selben Durchlauf zu verarbeiten.2 Überinfiltriertes Gewebe erweckt zuweilen einen gekochten Eindruck, ist spröde, brüchig oder staubtrocken. Auch unzureichend infiltrierte Proben lassen sich nicht schneiden. Biopsie- und größere Gewebeproben sollen deshalb separat, unter Verwendung spezifischer, der Probengröße angepasster Protokolle verarbeitet werden.3
Methode
Diese Studie wurde von Leica Biosystems' Content-and-Evidence-Team in einem kleinen unabhängigen Labor durchgeführt. Hier war man an der vollständigen Überprüfung der aktuellen Prozesse interessiert und suchte nach Empfehlungen, wie sich die Bearbeitungszeiten verkürzen und die Qualität der Ergebnisse steigern lassen. Nach einer initialen Beobachtungsphase konzentrierte sich das Team darauf, den Ablauf der Gewebeinfiltration im Labor in seinen Einzelheiten zu verstehen.
Ergebnisse
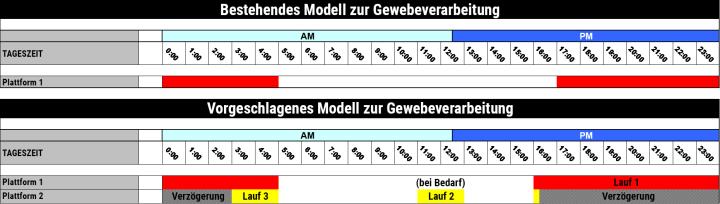
Diskussion
Das Content-and-Evidence-Team von Leica Biosystems analysierte den aktuellen Zeitplan des Labors für die Gewebeinfiltration. Man verwendete einen einzelnen Gewebeinfiltrationsautomaten mit einer Retorte, in dem über Nacht sämtliche Proben gemeinsam verarbeitet wurden. Diese Einzelcharge schloss unter anderem Biopsien sowie Standardproben aller Größen ein. Allerdings stellen diese Proben, etwa Biopsieproben und größere Gewebestücke oder fettarmes und fettreiches Gewebe, verschiedene Ansprüche an die Einwirkzeit der einzelnen Reagenzien. Werden sie dennoch in einer einzelnen Charge angesetzt, kann es zu einer Überprozessierung der Biopsien kommen, während größere Proben nicht vollständig infiltriert werden. Gewebe, das im ersten Anlauf nicht ausreichend infiltriert wird, muss aus dem Arbeitsablauf herausgenommen und in der nächsten Nacht erneut verarbeitet werden, wodurch sich die Bearbeitungszeit für diese Fälle um mindestens 24 Stunden verlängert.
Zum Abschluss der Studie wurde die Empfehlung ausgesprochen, die Verarbeitungsprotokolle zu trennen, um den verschiedenen Gewebetypen und -größen Rechnung zu tragen:
- Durchlauf Nr. 1 – über Nacht für 12 Stunden für die Routineproben
- Durchlauf Nr. 2 – tagsüber für 2 Stunden für Biopsien (optional)
- Durchlauf Nr. 3 – ein Durchlauf mit verzögertem Start für Biopsien, die am späten Nachmittag eingehen
Um die überlappenden Durchläufe im aktuellen Arbeitsablauf zu bewältigen, also nachts sowohl Routineproben als auch spät eingehende Biopsien verarbeiten zu können, wurde eine Umstellung auf zwei Retorten notwendig. Dies konnte entweder durch die Nutzung eines zweiten Infiltrationsautomaten mit einer Retorte oder eines einzigen Automaten mit Zwei-Retorten-Technologie erfolgen.
Fazit
Letztendlich ist es unverzichtbar, die Möglichkeit zur Umsetzung separater Verarbeitungsprotokolle zu schaffen, um für Proben verschiedener Größe eine qualitativ hochwertige Gewebeinfiltration zu erreichen. Die Ausführung eines kurzen Biopsieprotokolls während der Tagesschicht und der parallele Ansatz eines langen Protokolls und eines verzögerten Biopsiedurchlaufs über Nacht kann zu einer Erhöhung des Durchsatzes bei gleichzeitiger Verkürzung der Bearbeitungszeit für kleine Proben führen.
Ergebnisse und Hochrechnungen sind spezifisch für die Einrichtung, in der sie erzielt wurden, und spiegeln möglicherweise nicht wider, was in anderen Einrichtungen erreicht werden kann.
About the presenter

Ashley Troutman has been involved in Laboratory Medicine for more than 20 years in clinical, research and administrative capacities. He has worked in facilities of all sizes, from small community hospitals and private labs to large academic medical centers and corporate reference labs. He has extensive experience in laboratory science and management, specifically in anatomic pathology and immunohistochemistry. He has managed routine histology operations and has been part of the team to aid researchers in designing experiments using histologic techniques. These roles have allowed Ashley to lead work process implementation teams that saw success in scientific innovation as well as improving laboratory efficiency through areas of waste/cost reduction, process improvement and safety.
Referenzen
- Suvarna SK, Layton C, Bancroft JD. Bancroft’s Theory and Practice of Histological Techniques. 7th ed. London (UK): Churchill Livingstone Press, 2013.
- Carson, FL.„Histologic Fixation and Sample Processing“, in Brown RW (ed.) Histologic Preparations: Common Problems and Their Solutions. Northfield (IL): College of American Pathologists (US); 2009.
- Bancroft’s Theory and Practice of Histological Techniques: Expert Consult
Die Inhalte des Knowledge Pathway von Leica Biosystems unterliegen den Nutzungsbedingungen der Website von Leica Biosystems, die hier eingesehen werden können: Rechtlicher Hinweis. Der Inhalt, einschließlich der Webinare, Schulungspräsentationen und ähnlicher Materialien, soll allgemeine Informationen zu bestimmten Themen liefern, die für medizinische Fachkräfte von Interesse sind. Er soll explizit nicht der medizinischen, behördlichen oder rechtlichen Beratung dienen und kann diese auch nicht ersetzen. Die Ansichten und Meinungen, die in Inhalten Dritter zum Ausdruck gebracht werden, spiegeln die persönlichen Auffassungen der Sprecher/Autoren wider und decken sich nicht notwendigerweise mit denen von Leica Biosystems, seinen Mitarbeitern oder Vertretern. Jegliche in den Inhalten enthaltene Links, die auf Quellen oder Inhalte Dritter verweisen, werden lediglich aus Gründen Ihrer Annehmlichkeit zur Verfügung gestellt.
Vor dem Gebrauch sollten die Produktinformationen, Beilagen und Bedienungsanleitungen der jeweiligen Medikamente und Geräte konsultiert werden.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.
