Special Stains – Which One, Why and How? Part III: Microorganisms – Bacteria and Fungi

Define Microorganisms
Microorganisms are living organisms that include bacteria, fungi, protozoa, and viruses. They are so small that they require the aid of a microscope to visualize, sometimes even an electron microscope. Bacteria, fungi, and protozoa can be identified and classified with histochemical procedures, and viruses are generally identified with immunohistochemical procedures.
Introduction to Microorganisms – Bacteria
Microorganisms consist of single-celled organisms a few micro millimeters in size. Three that are common to humans are mycobacterium tuberculosis, mycobacterium leprae, and helicobacter pylori.
MYCOBACTERIUM TUBERCULOSIS
Causes tuberculosis, is non-motile and rod-shaped, and is characterized and identified by a property known as "acid-fast" meaning that once absorbed, the dye used to stain the bacilli should not be removed when incubated in an acid rinse. The preferred method of identification is an AFB stain, otherwise called an acid-fast bacteria stain.
Mycolic acid is the mechanism responsible for the acid-fast property. The walls of the bacteria produce this waxy substance called mycolic acid. This acid links with substances within the cell walls of the bacteria, making them resistant to decolorization with acid alcohol treatment. The most common choices of AFB stains are Kinyouin’s carbol fuchsin or Ziehl-Neelsen carbol fuchsin. The solutions, procedures, and results are similar for both.
Kinyouin’s Carbol Fuchsin
The Process
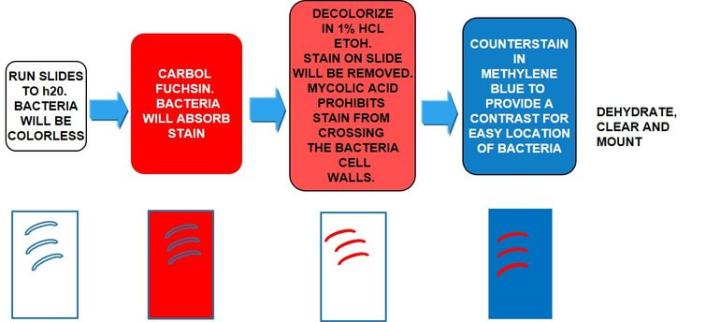
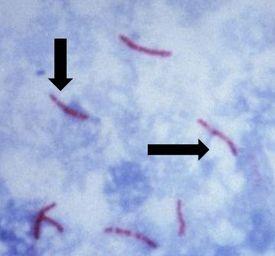
The Results
The Protocol
Incubation times may vary per manufacturer
|
1. |
Bring slides to water |
|
|
2. |
Carbol fuchsin (room temp.) |
30 to 60 min |
|
3. |
Application of heat to 60c can shorten incubation times |
|
|
4. |
Rinse well in running water |
5 min |
|
5. |
1% HCL acid alcohol (70% alcohol) |
10 sec |
|
6. |
Rinse well in running water |
2 min |
|
7. |
Methylene blue to the desired color |
30 to 40 sec |
|
8. |
Rinse briefly in running water |
15 sec |
|
9. |
Dehydrate quickly through alcohols |
|
|
10. |
Clear and mount |
Troubleshooting the AFB stain:
It is very important to rinse well after carbol fuchsin to remove excess solution. Make sure the water level covers the entire slide all the way over the top. Residual stain can streak through the tissue muddying the results. Decolorization will occur quickly. Incubate in HCL/70% EtOH to the point that the glass slide is clean, and the tissue looks slightly pink. After decolorization, run in water, washing very well, making sure not to leave any acid/EtOH on the slide. With methylene blue, less is more. If you overstain in this counterstain, the AFB bacilli can be masked. There is a fine line between too much methylene blue and too little. If there is too much, it may not readily wash out and mask the bacilli. If there is too little, it may all wash out, leaving no contrast. Trial and error and experience is the best answer.
As stated above, methylene blue may wash out quickly in alcohols, so make sure to start with 100% for dehydration and run slides quickly through 2 changes just to remove water from the slide.
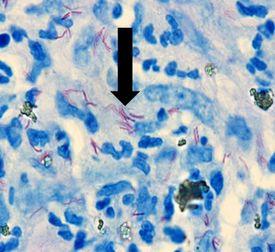
MYCOBACTERIUM LEPRAE
causes leprosy, has acid-fast properties similar to M. Tuberculosis but much less effective. The preferred stain for demonstration is Fite’s Faraco.
Fite’s Faraco
The Process

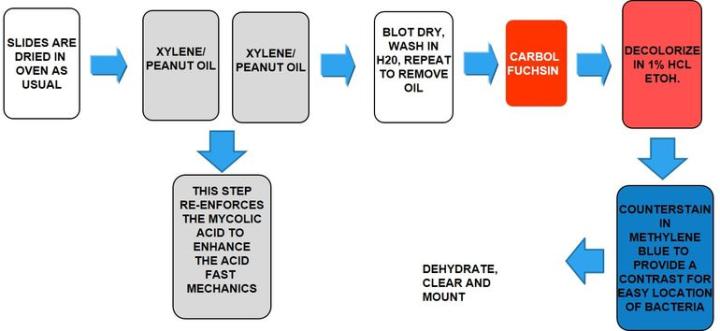
The Results
The Protocol
Incubation times may vary per manufacturer.
|
1. |
Dry slides in oven |
|
|
2. |
Incubate in a 50/50 mixture of xylene/peanut oil |
15 min |
|
3. |
Repeat with a second 50/50 mixture |
15 min |
|
4. |
Blot dry, wash in water, repeat to remove oil |
5 min |
|
5. |
Fite’s carbol fuchsin (room temp.) |
20 min |
|
6. |
Rinse well in running water |
|
|
7. |
1% HCL/70% alcohol |
1 to 5 sec |
|
8. |
Rinse in running water |
|
|
9. |
Methylene blue |
10 to 20 sec |
|
10. |
Rinse quickly in running water |
|
|
11. |
Blot dry, do not place in alcohols, clear and mount |
Troubleshooting the Fite's – Faraco:
If slides are accidentally placed in xylene and alcohols per a normal dewaxing protocol, cut fresh slides and start over. Follow the same guidelines as the Kinyouin’s carbol fuchsin for best results.
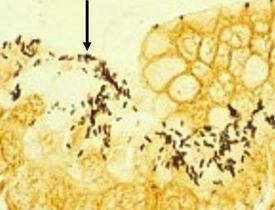
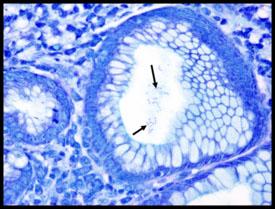
HELICOBACTER PYLORI
is spiral-shaped and is associated with gastric inflammation, peptic ulcers, and gastric carcinoma. They have argyrophilic properties and cannot absorb or reduce silver. The preferred stains for demonstration are Warthin Starry silver or one of many modifications, Giemsa, and immunohistochemistry procedures (most sensitive).
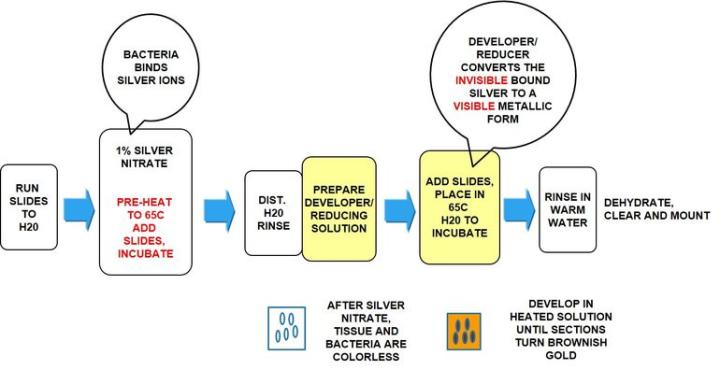
Warthin Starry Silver
The Process
The Results
The Protocol
Incubation times may vary per manufacturer guidelines
Note: the Warthin Starry silver stain is used to demonstrate many varied microorganisms, not just H. Pylori. For this reason, you may find protocols that vary from the protocol specific for H. Pylori below.
|
1. |
Bring slides to water |
|
|
2. |
Rinse in DI water |
|
|
3. |
0.5% to 1% silver nitrate – preheated to 65°C |
3–5 min |
|
4. |
Developer/reducing solution |
1–5 min |
- Place slides in Coplin jar containing solution
- Add Coplin jar to preheated 65°C water bath
- Incubate until tissue section obtains brownish/gold color

|
5. |
Rinse in warm water |
2–3 min |
|
6. |
Dehydrate in absolute alcohols |
|
|
7. |
Clear and mount |
Troubleshooting the Warthin Starry silver stain for H. Pylori
Troubleshooting the Warthin Starry silver stain for H. Pylori:
The Warthin Starry can be a difficult stain to master. Make sure to follow all manufacturer’s directions for mixing solutions. Use clean glassware and DI water when called for. Preheating a water bath for the silver and developer/reducer is important. Silver should be at 65°C when slides are loaded. As the developer/reducer warms, the tissue will start to develop a gold-brown color when nearing completion. Agitation in the developer/reducer solution will create a more evenly stained slide.
Note: Microwave heating can be substituted for water bath heating. Times should be adjusted.
Giemsa
Giemsa is a polychromatic stain made up of dyes with different hues.
The Process
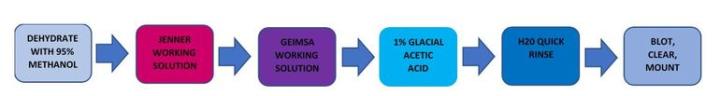
The Results
The Protocol
Incubation times may vary per manufacturer guidelines
|
1. |
Dehydrate with 95% Methanol |
|
|
2. |
Jenner working solution |
10 min |
|
3. |
Giemsa working solution |
45 min |
|
4. |
1% glacial acetic acid water |
10 sec |
|
5. |
Quick rinse in running water |
|
|
6. |
Blot well, clear, and mount |
Troubleshooting the Jenner-Giemsa for H. Pylori:
Jenner and Giemsa are two very unstable solutions and should be made fresh for each use. Filter both before using. The glacial acetic step is critical. Staining too short will cause poor differentiation in the cells. Staining too long will wipe out most of the staining. Blot instead of dehydrating through alcohols as all the blue in the h. Pylori will wash out.
A comparison of three procedures for demonstration of H. Pylori.
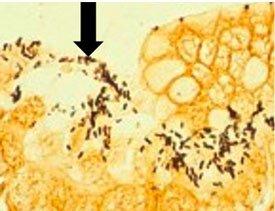
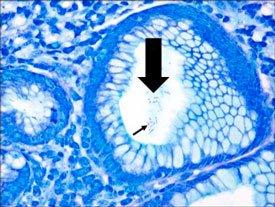
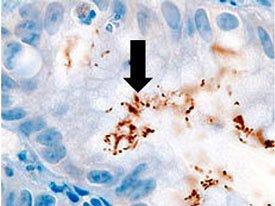
Introduction to Microorganisms - Fungi
Fungi consist of both single and multicellular organisms with distinct nuclei and cell walls. Three common fungi known to cause diseases in humans are cryptococcus neoformans, histoplasma capsulatum, and pneumocystis carinii.
CRYPTOCOCCUS NEOFORMANS
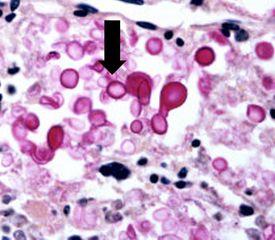
Lives in the environment throughout the world and can cause meningitis in humans. Infections usually occur in immunocompromised individuals. Capsules are clearly demonstrated by the mucicarmine stain as well as by a methenamine silver procedure (see process below).
Mucicarmine
The Process
The Results
The Protocol
Incubation times may vary per manufacturer guidelines
|
1. |
Run slides to water |
|
|
2. |
Working Wiegert’s hematoxylin |
5–10 min |
|
3. |
Rinse in running water |
|
|
4. |
Working mucicarmine solution |
20–30 min |
|
5. |
Rinse in running water |
|
|
6. |
Metanil yellow |
1–3 min |
|
7. |
Rinse briefly in running water |
|
|
8. |
Dehydrate, clear, and mount |
Troubleshooting the Mucicarmine stain:
Follow manufacturer’s instructions for making working mucicarmine. Remember to dilute according to instructions and use appropriate water for dilution (distilled vs. tap). Use fresh Wiegert’s. If your Wiegert’s is not purple but more brownish, it has broken down and your nuclei will not stain properly. Working Wiegert’s might last a full day but normally not overnight.
HISTOPLASMA CAPSULATUM
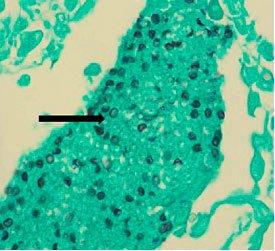
causes histoplasmosis. Infection occurs from breathing in these spores, which results in flu-like symptoms. If left untreated, it can disseminate throughout the body and cause death. It is more common in immunocompromised patients. It can be demonstrated by the Gomori’s methenamine silver stain.
PNEUMOCYSTIS CARINII
was previously classified as protozoa but is now considered a fungus-based on nucleic acid and biochemical analysis. It can lead to a fatal pneumonia in immunosuppressed patients. It can be demonstrated by the Gomori’s methenamine silver stain.
Gomori’s methenamine silver
Gomori’s methenamine silver relies on cells that have an argentaffin property. It can reduce invisible silver ions to visible metallic silver without an external aid and is opposite of argyrophilic as seen in H. Pylori.
The Process
The Results
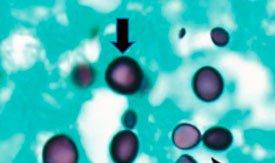
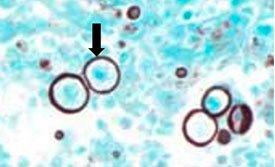
The Protocol
|
1. |
Bring slides to water |
|
|
2. |
Oxidize in chromic acid |
10 min |
|
3. |
Rinse well in running water |
3 min |
|
4. |
Sodium bisulfite |
1 min |
|
5. |
Rinse in running water |
|
|
6. |
Incubate in methenamine silver solution at 60c. |
10 – 20 min |
|
7. |
Incubation time is determined by rate of impregnation |
|
|
8. |
Rinse in DI water |
|
|
9. |
Gold chloride |
1 – 2 min |
|
10. |
Rinse in running water |
|
|
11. |
Sodium thiosulfate |
1 min |
|
12. |
Rinse in running water |
|
|
13. |
Counterstain in light green |
30 - 40 sec |
|
14. |
Rinse in running water quickly |
|
|
15. |
Dehydrate, clear, and mount |
Troubleshooting the GMS:
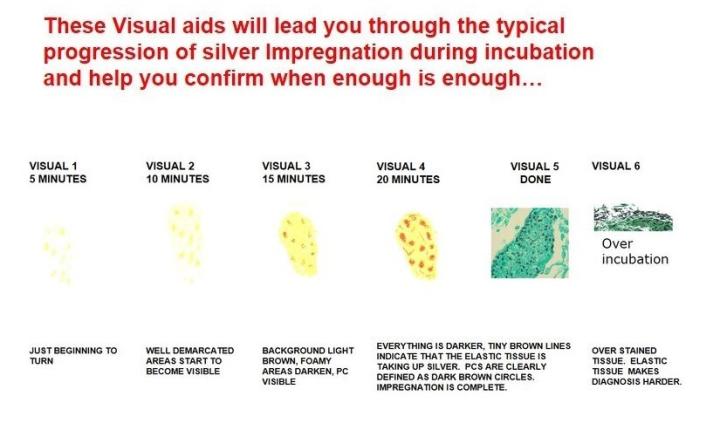
Chromic acid should be changed often to promote complete oxidation and is dependent on the number of slides run. Keep the vessel closed when not in use. Impregnation times (if done manually) require some trial and error, depending on the heating method used (water bath vs. Microwave oven). Do not overstain with light green as this may mask the organism you wish to demonstrate.
GMS for Pneumocystis
About the presenter

Carolyn Doan received her ASCP registration in 1969 after completing classes at Georgia State University and St Joseph school of Histotechnology in Atlanta, Georgia. For 40 years she shared her passion for histology in research (at Yerkes Primate Research Center), in the clinical world (managing the Pathology Department at Florida Hospital in Orlando and serving on the board of the Florida State Licensure Task Force and as president of the Florida State Society for Histotechnology), and in industry (as a Sales executive, Marketing Manager and North American staining sales specialist). Since her retirement in 2013, she founded Creative Histology Consulting.
References
Geoffrey Rolls. “101 Steps to Better Histology – A Practical Guide to Good Histology Practice”. Pathology Leaders/articles
Shaikh, Dr. Imran, “Special Stains in Histopathology.” Kem Hospital, 2012
Ellis, Roy. IMVS Division of Pathology, The Queen Elizabeth Hospital, Woodville Road, South Australia
Wikipedia encyclopedia – Pneumocystis Carinii
Kerr, DA: Improved Warthin Starry Method for Tissue Sections; Am J Clin Pathol; 1938, 8:63-67
Dacie and Lewis, Practical Hematology, 12th edition, 2017
ncbi.nlm.nih.gov/pubmed/8173783 - The Identity of Pneumocystis Carinii
Sheehan/Hrapchak. “Theory and Practice of Histotechnology.” Microorganisms. 2nd edition, 13: 233-251
Derm101.com Ackerman, A. Bernard (2000)
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2025 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.