
Assessing Image Quality: A Comparative Multi-assessment Evaluation of Aperio GT 450 DX
The number of practising anatomic pathologists is falling globally, and pathology departments need to find new ways to do more with less. Digital Pathology can provide a flexible platform to improve workflow efficiency while also ensuring patient safety and quality medical care. The key priority of any successful digital pathology implementation is to ensure that when a pathologist views digital slide images, they are as confident making the diagnosis as they are when looking at glass slides on a microscope.
The Aperio GT 450 DX is an automated, high capacity slide scanner made by Leica Biosystems. The highperformance objective included with the Aperio GT 450 DX is specifically designed to maximise field of view for high speed digital pathology scanning. To investigate the quality of slide images generated on the Aperio GT 450 DX, seven pathologists at two different sites rated the quality of images created by the Aperio GT 450 DX.
Method
The study, conducted by the Leica Biosystems Content & Evidence Team, occurred at two independent sites in Europe: a University Medical Center in the Netherlands, and a Cancer Institute in Italy. The sites were selected based on their different levels of experience with digital pathology, from minimal (Site 1) to routine use for diagnostics (Site 2). Each site generated their own set of 30 digital slide mages using the Aperio GT 450 DX, representative of their site’s daily work. At Site 1, four pathologists viewed a shared set of 30 digital slide images created within their facility, while Site 2 had three pathologists view a shared set of digital slide images created within their facility. Since the pathologists participating in the study regularly read multiple tissue types, the digital slide images included a wide variety of tissue, including: stomach biopsies, bone marrow biopsies, skin biopsies, kidney biopsies, liver resections, thyroid esections, lung resections and ovarian resections. Additionally, multiple staining techniques were utilised, including: haematoxylin and eosin (H&E), Grocott’s Methenamine Silver (GMS), Giemsa, and several immunohistochemical stains (ER, PR, CD10, SOX-10).
Every image was assessed and scored by each pathologist using a 4-point scoring system:
- Poor Image Quality: Major issues seen, frequently blurry
- Moderate Image Quality: Both minor and major issues
- Good Image Quality: Benchmark for adequacy. Some minor image quality issues
- Excellent Image Quality: Same as microscope or better
The scores for each pathologist were analysed, averaged individually and within each site. Score distributions and means were then compared with ANOVA between pathologists. No comparisons were made between the two sites.
Results
At Site 1, the average image scores from each pathologist ranged from 3.73 to 3.87, well above the 3.00 benchmark for adequacy (Figure 1).
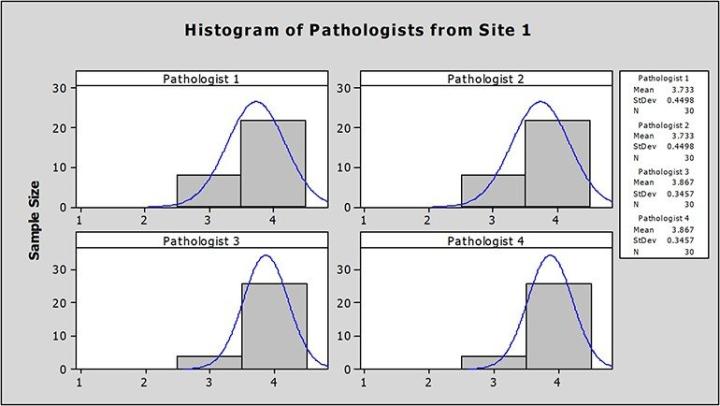
At Site 2, the average image scores from each pathologist ranged from 3.53 to 3.76, also well above the 3.00 benchmark for adequacy (Figure 2).
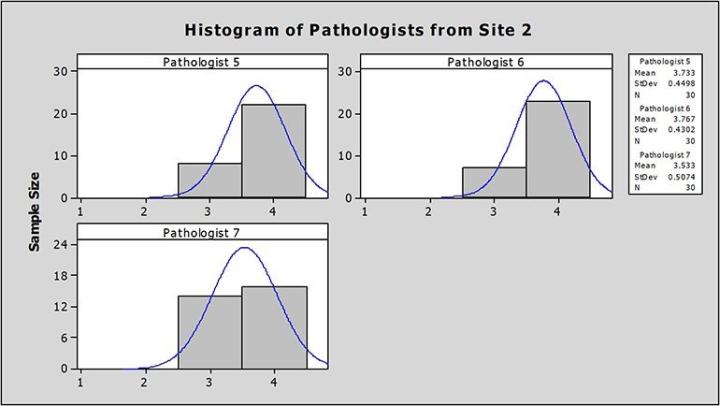
Using ANOVA, there was no significant difference between the mean quality scores from pathologists at each site (Site 1 p= 0.35; Site 2 p=0.11).
Discussion
The data demonstrated that the Aperio GT 450 DX generated high quality slide images, as judged by practising pathologists. The majority of digital slide images at Site 1 (80% of 120 assessments) and Site 2 (68% of 90 assessments) were given the highest score, excellent quality, with 100% of the digital slide images rated at the benchmark for adequacy. We did not observe a correlation between mean quality scores and the experience a site had with digital pathology. This suggests that prior experience with digital slide images is not requisite for recognition of quality images.
Conclusion
Independent of a pathologist’s previous level of experience viewing digital slide images, analysis of the data suggests a high level of confidence when looking at digital slide images from the Aperio GT 450 DX as when looking at glass slides on a microscope.
Projections and Realised Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
For In Vitro Diagnostic Use. The clinical use claims described for the products in the information supplied have not been cleared or approved by the U.S. FDA or are not available.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
참조 문헌
This reference document is presented as a service to health care professionals by Leica Biosystems and has been compiled from available literature. Although every effort has been made to report faithfully the information, Leica Biosystems cannot be held responsible for the correctness. This document is not intended to be, and should not be construed as medical advice. For any use, the product information guides, inserts and operation manuals of the various drugs and devices should be consulted. Leica Biosystems and the editors disclaim any liability arising directly or indirectly from the use of drugs, devices, techniques or procedures described in this reference document.
Leica Biosystems 콘텐츠는 Leica Biosystems 웹사이트 이용 약관의 적용을 받으며, 이용 약관은 다음에서 확인할 수 있습니다. 법적고지. 라이카 바이오시스템즈 웨비나, 교육 프레젠테이션 및 관련 자료는 특별 주제 관련 일반 정보를 제공하지만 의료, 규정 또는 법률 상담으로 제공되지 않으며 해석되어서는 안 됩니다. 관점과 의견은 발표자/저자의 개인 관점과 의견이며 라이카 바이오시스템즈, 그 직원 또는 대행사의 관점이나 의견을 나타내거나 반영하지 않습니다. 제3자 자원 또는 콘텐츠에 대한 액세스를 제공하는 콘텐츠에 포함된 모든 링크는 오직 편의를 위해 제공됩니다.
모든 제품 사용에 다양한 제품 및 장치의 제품 정보 가이드, 부속 문서 및 작동 설명서를 참조해야 합니다.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.