
Tecniche speciali di colorazione per la valutazione delle mucine

Special Stain Techniques for the Evaluation of Mucins
Special stains that are used for the evaluation of mucins, mucin-like molecules and other carbohydrate containing macromolecules remain in demand and are utilized frequently in the histology laboratory. This article is intended as a basic review of the mechanisms of action of the most commonly used mucin special stains including Alcian blue, mucicarmine, colloidal iron and the periodic acid Schiff technique. In order to understand these mechanisms, however, it is helpful to first review the basic structure and chemistry of the mucins.
Introduction
Mucins are high molecular weight glycoproteins that are found dispersed throughout the epithelia of the gastrointestinal, respiratory and reproductive tract. Mucins are composed of a central protein core with multiple chains of carbohydrates (polysaccharides) attached. The carbohydrate content of the mucin molecule may account for 60-80% of the molecular weight of the molecule. The protein core contains a high content of the amino acids' serine and threonine. A defining structure of the mucins is the presence of tandem repeats of specific amino acid sequences within the protein core. From a molecular perspective, mucins are categorized into distinct families (Muc 1, Muc 2, Muc 3, etc.) based upon differences in the sequence and size of the tandem repeats.
There are a number of other glycoprotein molecules which share structural similarities with the mucins and often are confused with mucins. Proteoglycans are high molecular weight glycoconjugate complexes that are found in high concentrations within the extracellular matrix as well as connective tissues. In older literature, proteoglycans frequently are referred to as connective tissue mucins. However, the structure, (particularly the protein core of the proteoglycans), is different and distinct from that of mucins.
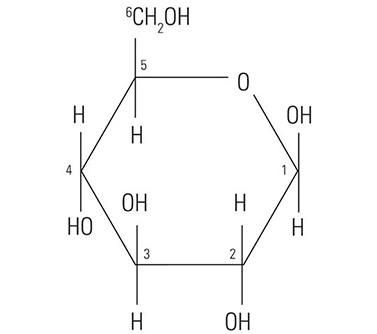
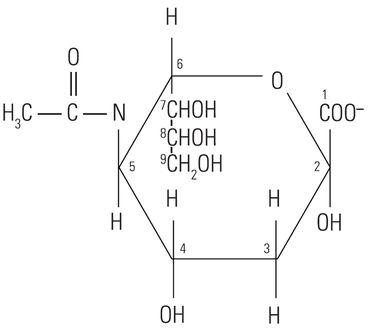
The protein core of the mucins has little or no bearing on the histochemical reactivity of the mucins with the dyes used in special stain techniques. In fact, the histochemical reactivity is dependent largely upon the carbohydrate composition of the mucins. The word “carbohydrate” was coined over a hundred years ago to describe a diverse family of molecules of the general formula Cn(H20)n. The word “carbohydrate” actually is descriptive of the 1:1 ratio of carbon molecules to water (hydrate). The glucose molecule (Figure 1) demonstrates the structure of the typical carbohydrate monosaccharide. Other similar monosaccharides are mannose and galactose. These molecules are not charged as they demonstrate no ionizable groups under normal conditions. In contrast other monosaccharides may contain acidic groups such as carboxyl (COOH) and sulphonic (SO3H) groups that are capable of ionization to confer an overall negative charge on the molecule. The carboxylated monosaccharide N-acetylneuraminic acid which is commonly known as sialic acid is shown in (Figure 2). The presence of these types of functional groups defines in part the potential reactivity with histochemical stains.
From a histochemical perspective, mucins can be placed in one of two broad categories based upon the chemistry and composition of the carbohydrate component of the mucin. The charged or “acid” mucins contain carbohydrates with carboxylate (COO-) or sulphonate (SO3) groups. Both of these groups are ionized at a physiologic pH to produce an overall negative charge on these mucin molecules. The carbohydrate chains of neutral mucins lack acidic groups and thus carry no net charge. The neutral mucins can be found primarily in the surface epithelia of the stomach, Brunner’s glands of the duodenum and in the prostatic epithelium. The acid mucins are found widely distributed throughout the gastrointestinal tract and the respiratory tract.
The typical mucin special stains contain cationic (positively charged) dye molecules in solution at a specific pH. This is true in the case of mucicarmine, Alcian blue as well as the older metachromatic techniques that utilized such dyes as azure A or toluidine blue. The cationic dye molecules bind via electrostatic forces to the anionic carboxylated or sulfated polysaccharide chains of the mucin molecules.
Mucicarmine
Mucicarmine is one of the oldest techniques for the detection of mucins. Although not as commonly used as it was, mucicarmine is still a valuable technique for the evaluation of acid mucins particularly those of the gastrointestinal tract. In addition, this technique is also useful for staining the capsule of the fungus Cryptococcus neoformans.
The active dye molecule found in the mucicarmine stain is a chelate complex formed between cationic aluminum ions and carminic acid. Carminic acid is a natural dye molecule that is isolated from the dried bodies of female Coccus cacti insects. The aluminum cations confer an overall positive charge to the large carmine complex. Although the exact mechanism(s) by which this complex selectively stains mucins is unknown, evidence suggests that it is by electrostatic attraction to the anionic groups of acid mucins. This theory is supported by the observations that tissue sites that contain an abundance of neutral mucins demonstrate little or no staining while the sites of the gastrointestinal tract that are known to contain acid mucins typically stain strongly with mucicarmine.
Alcian Blue
Alcian blue is a large planar phthalocyanine molecule with a copper atom in the center. The molecule also contains four basic isothiouronium groups which carry a positive charge. The positive charge imparted by these groups results in the attraction of the Alcian blue dye molecules to the anionic sites in mucin molecules. Like mucicarmine, Alcian blue does not stain neutral mucins.
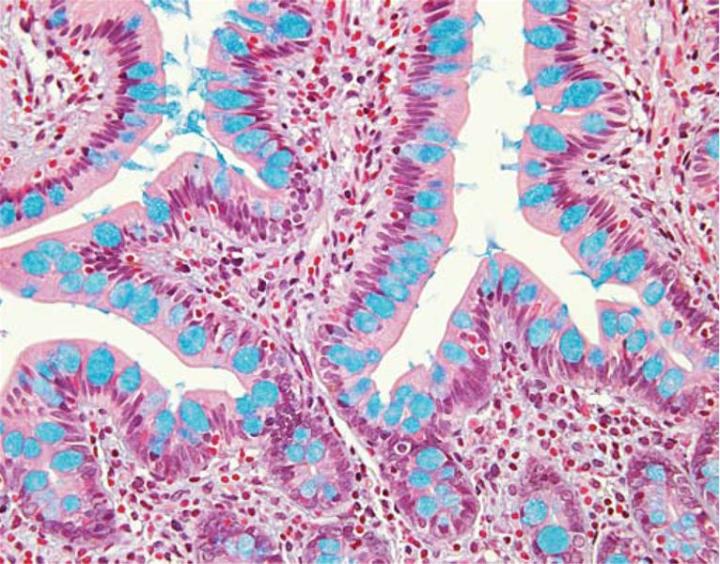
Varying the pH of the Alcian blue solution is a useful means for further characterization of the subtypes of acid mucins present in a tissue specimen. All acidic mucins whether carboxylated or sulfated will ionize at a pH of 2.5 to produce anionic groups (COO, SO3). Thus the standard Alcian blue – pH 2.5 stains all acidic mucins (Figure 3).In contrast, mucins that contain predominately carboxylated carbohydrates will stain strongly with Alcian blue – pH 2.5 but not with Alcian blue at a pH of 1.0. The carboxyl groups do not ionize at this lower pH and as a result the mucins will display neutral characteristics. The more acidic sulphonic groups are capable of ionization at a pH of 1 and therefore stain at this pH.
Colloidal Iron
The colloidal iron technique is based upon the attraction of positively charged iron ions (ferric cations) for the negatively charged carboxylate and sulfate group endings in acidic mucins. The bound ferric ions are detected or visualized by subsequent treatment with potassium ferrocyanide to form bright blue deposits of ferric ferrocyanide or Prussian blue. While the colloidal iron technique is an extremely sensitive method for the detection of acid mucins, it is not as frequently used as other techniques such as Alcian blue. However, like Alcian blue the colloidal iron technique may be used in combination with the periodic acid also known as the Schiff technique.
The Periodic Acid – Schiff (PAS) Technique
The PAS technique is perhaps the most versatile and widely used of the techniques for the demonstration of glycoproteins, carbohydrates and mucins. Unlike the other techniques described thus far, the PAS technique also recognizes neutral mucins. The reactivity of the PAS technique is not based upon the presence of acidic groups among the polysaccharides but instead upon the structure of the monosaccharide units.
The PAS technique is based upon the reactivity of free aldehyde groups within the monosaccharide units with the Schiff reagent to form a bright red magenta end product. The initial reaction in the PAS technique is the oxidation of hydroxyl (OH) groups attached to adjacent carbon atoms. These groups are referred to as 1, 2 glycols. Oxidation of these glycols results in the formation of Schiff reactive aldehyde groups. In most protocols, a 0.5% to 1.0% solution of periodic acid is used as the oxidant. The intensity of the color that develops following treatment with the Schiff reagent is proportional to the concentration of reactive 1-2 glycol groups within the tissue. The PAS technique is particularly sensitive for the detection of neutral mucins as well as acid mucins that contain significant quantities of sialic acid. In addition to mucins, the PAS technique is also widely used for the detection of glycogen and various glycoproteins. The PAS technique is particularly valuable for the visualization of basement membranes due to the presence of Schiff reactive glycoproteins within these structures.
Alcian Blue / PAS
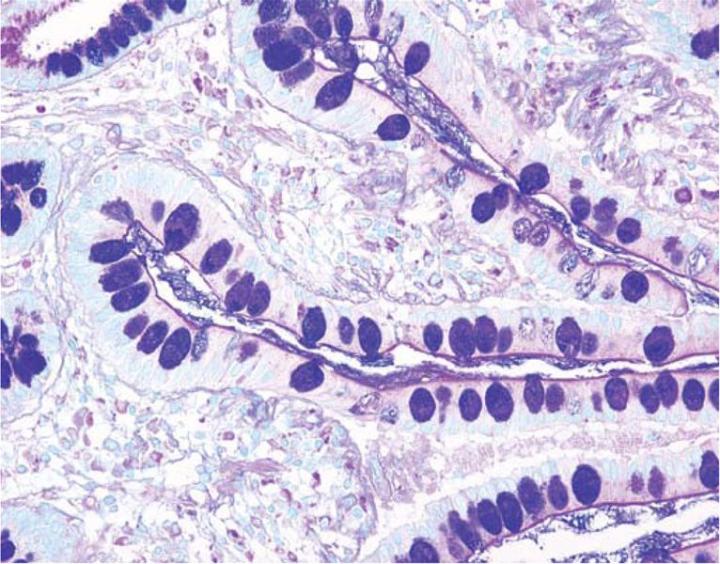
The combination of the Alcian blue and the PAS techniques can be used as a means of distinguishing neutral mucins from acid mucins. In most protocols, sections are stained with the standard Alcian blue (pH 2.5) method followed by the PAS technique. The Alcian blue at a pH of 2.5 will stain all acid mucins deep blue but will not color the neutral mucins. The subsequent application of the PAS technique will stain the neutral mucins bright magenta. Tissues or cells that contain both neutral and acidic mucins may demonstrate a dark blue or purple coloration (Figure 4). The combined application of Alcian blue and PAS is useful for several reasons. Changes in the distribution or pattern of expression of neutral and acid mucins are indicative of certain pathological conditions. In addition, the combined Alcian blue/PAS technique is perhaps the most sensitive or comprehensive means for detection of mucins as all mucins should react regardless of the charge nature of the mucin.
Figures
About the presenter

Russell Myers acquired his Ph.D. in endocrine physiology from the Medical College of Georgia. Russell Myers is responsible for design and development of reagents and consumables used in the histology laboratory.
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.