Actualités Leica Biosystems
Updates about Leica Biosystems.
Filter by post category:

Research-use-only RNA-ISH probes now available for COVID-19 on our automated platform Leica Biosystems, in partnership with Bio-Techne Corporation, announces the automation of RNAscope™ COVID-19 probes’ on BOND RX*, its staining platform, for research use only. Researchers now have the capability…

Leica Biosystems, a global leader in pathology workflow solutions, announced the European launch of its Aperio GT 450 DX digital pathology scanner. This next generation imaging system was unveiled at the highly successful Leica Biosystems Digital Pathology Virtual Summit and is already installed at…

VISTA, CA April 24, 2020 – Leica Biosystems, the global leader in pathology workflow solutions, today announced it has received additional notification from the U.S. Food & Drug Administration (FDA) about its intention to exercise enforcement discretion when the Aperio WebViewer, a web-based…
VISTA, CA, April 3, 2020 – Leica Biosystems, the global leader in pathology workflow solutions, today announced it has received notification from the U.S. Food & Drug Administration ( FDA ) that its Aperio ImageScope DX Viewer with images acquired on the Aperio AT2 DX Scanner can be used for…

VISTA, Calif.—Mar 2, 2020—Leica Biosystems, a globally recognized cancer diagnostics and workflow solution leader, today announced a significant development for anatomical pathology laboratories by bringing together its Aperio AT2 DX System—digital pathology whole slide imaging—with Aperio Path DX…

VISTA, CA –December 2nd, 2019 – Leica Biosystems, the global leader in pathology workflow solutions, and the international medical imaging IT and cybersecurity company, Sectra, announce a collaboration to seek a combined 510(k) clearance from the U.S. Food & Drug Administration (FDA) in the…

International panel of pathologists drive forward-thinking agenda on role of pathology in transforming cancer diagnostics and improve patient care London, UK – Nov. 13, 2019. On International Pathology Day, Leica Biosystems, a world-leading cancer diagnostics company, today announced the launch of…
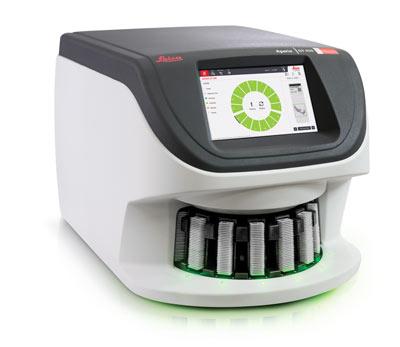
VISTA, CA – Aug 9, 2019 – Leica Biosystems, the global leader in pathology workflow solutions, announced today that it has launched the Aperio GT 450, its next generation digital pathology scanner. The Aperio GT 450, labeled as research use only, offers continuous rack loading with priority…

VISTA, CA –May 29, 2019 – Leica Biosystems, the global leader in pathology workflow solutions, announced that it has received clearance from the U.S. Food and Drug Administration (FDA) to market its Aperio AT2 DX System for clinical diagnosis in the U.S. A multi-center study supporting this…

VISTA, CA – January 7th, 2019 – Leica Biosystems, the global leader in pathology workflow solutions announced today that the US Patent Office has granted it a patent for RTF, its breakthrough “Real-Time Focusing” technology (US patent 9,841,590). This will enable high volume, extremely fast line…