
Steps to Better Grossing

From patient to pathologist, preparing tissue specimens for histological examination requires care, skill and sound procedures. This guide provides practical advice on best practice techniques and simple ways to avoid common errors.
Tips for better grossing are highlighted in this guide. We hope each step provides a valuable reminder of good histology practice and also helps with troubleshooting when unacceptable results do occur.
Check Fixation Status
Specimens are dealt with promptly (especially large specimens that may otherwise be inadequately fixed).
No consideration given to optimizing the fixation of problem specimens.
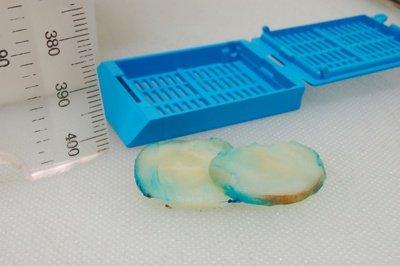
Prepare Thin Slices
Care is always taken to prepare uniform, thin slices from large specimens (3–4 mm maximum thickness). This is particularly important with dense tissues.
Slices are sometimes 6 mm (or more) thick and are often uneven.
Avoid Specimen Trauma
Care is taken to avoid traumatizing delicate specimens, particularly those that are incompletely fixed (handle carefully, do not crush, always use sharp blades).
Specimens are handled roughly without any consideration as to their state of fixation. Sometimes blunt blades are used in dissection.
Avoid Cross-contamination
Each specimen is handled on a clean surface avoiding the possibility of specimen-to-specimen contamination.
Sometimes the surface of the cutting board is not properly cleaned between specimens. This is of concern when the same specimen types are cut up one after the other. You do not want to have carry over from a specimen that is malignant to one that is benign.
Take Care with Biopsy Pads
Fresh or incompletely fixed specimens are not placed between foam biopsy pads, particularly needle-core specimens (biopsy pad artifact is avoided).
Sometimes small, fresh or incompletely fixed specimens are placed between biopsy pads, put into a cassette and then fixed. This can produce a characteristic artifact.
Choose Appropriate Cassettes
Choose appropriate cassettes for the specimen type being processed. Tissue fragments shrink during processing and, if cassette perforations are too large, fragments may escape into processing reagents or, worse still, transfer over to another specimen.
A “one size fits all” approach is used when placing specimens into cassettes.
Avoid Overloading Cassettes
Cassettes are never overloaded with tissue thus allowing ready access to processing reagents and preventing distortion of specimens. If the volume of tissue is too great a second cassette is used.
Cassettes are often crammed full of tissue thus preventing access of processing reagents. Sometimes specimens are distorted in the process.

Clearly Label Cassettes
Cassettes are always clearly labeled. Accurate identification of specimens is of paramount importance.
Sometimes it is difficult to read the labels on cassettes. A bit of guess work may be required.
About the presenter

Geoffrey Rolls is a Histology Consultant with decades of experience in the field. He is a former Senior Lecturer in histopathology in the Department of Laboratory Medicine, RMIT University in Melbourne, Australia.
Related Content
Leica Biosystems Knowledge Pathway content is subject to the Leica Biosystems website terms of use, available at: Legal Notice. The content, including webinars, training presentations and related materials is intended to provide general information regarding particular subjects of interest to health care professionals and is not intended to be, and should not be construed as, medical, regulatory or legal advice. The views and opinions expressed in any third-party content reflect the personal views and opinions of the speaker(s)/author(s) and do not necessarily represent or reflect the views or opinions of Leica Biosystems, its employees or agents. Any links contained in the content which provides access to third party resources or content is provided for convenience only.
For the use of any product, the applicable product documentation, including information guides, inserts and operation manuals should be consulted.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.



