Glass Slides: Science of Surface Modification

Andrew will explain how glass is made and how we change its surface, so it is not inert.He will also review the common factors contributing to specimen loss and describe the benefits and dangers of the adhesives currently used. These modifications dictate the different glass surface properties such as hydrophobicity and hydrophilicity.
학습 목표
- Explain the need for coated glass slides
- Recall the different adhesives used in for slide coating
- Describe the basic chemistry of slide coating
- Memorize the terms hydrophobicity, hydrophilicity,wettability, contact angle
웨비나 전사
ANDREW LISOWSKI, M.S., HTL (A.S.C.P.):
Hello everybody. I’m excited to talk about the glass slides and more than that, the science of surface modification, something that is elusive. Very few people have done research on that and there are few papers on it, but histologists are interested in how glass is made, how glass types differ from one another, and how you coat slides.
I will talk about why we need coated glass slides; the different adhesives used in slide coating; some basic chemistry processes of slide coating that you can explain to your customers; and hydrophobicity, hydrophilicity, wettability and contact angle.
What is the microscope glass slide? It is a thin, flat piece of glass that is made from the highest-quality glass and typically 75 x 25 mm and 1 mm thick or 3 x 1-inch x 1 mm. Some slides are thicker, but most people use 1 mm thick. The slides are used to hold a specimen, so there could be a stain for examining under the microscope. The glass slides have to be ground and polished for safe handling. They also have a frosted area painted with a special patient that holds the information of the specimen for labeling with a pencil, pen or automated printer.
How is glass made?
How are the glass slides made? There are a variety of videos on YouTube. It is amazing. Basically, the glass slide is made from sand and a couple of chemicals, limestone and soda. Sand, sodium carbonate and calcium carbonate are heated to 1700 C or over 3000 F, which is hotter than molten lava. This takes about 10 hours, at which time the sand becomes a liquid. Then you pour the liquid sand on a thin liquid tin, and it becomes glass. It’s a process that takes quite a bit of time, but this is how glass has been made for centuries. It was discovered when volcanoes erupted and at that temperature, sand would melt and create quartz or pieces of glass.
What is the difference between a glass to drink wine and glass we use in a histology lab? As indicated in the chart on the slide, borosilicate glass is what we call premium. A glass made for a laboratory would not have additives. Even higher temperatures are needed to melt the sand and because you’re not adding anything, the glass is cleaner and has better optical and thermal properties. This glass would be more expensive, cleaner, and used for sophisticated pieces of laboratory items. Most glass on the market is the inexpensive kind, but if you’re working on a sophisticated molecular application, you might use the borosilicate glass, which has the important optical and thermal properties.
Glass is chemically inert. This picture shows the silica atom in white, which binds with the four oxygen atoms. Each oxygen atom binds with one silica atom. Glass does not make chemical bonds because it is so solid, and all the bonds are taken. You must almost force a bond with anything at all. Crystalline silica exists in nature in quartz, sand, gravel, clay or granite. Non-crystalline silica would be your average window glass.
Why do we even talk about the glass? Because it will hold a piece of tissue on top of it that will be stained later on. If you use the plain glass with no chemicals on top of it and not modified glass, you are risking losing the tissue. If you place tissue on plain inert glass, the only forces that exist are the electrostatic forces. These are the electrical charges and so there’s no chemical bond that is formed between that glass and the specimen. This is why people who use non-coated slides or non-charged slides would use the temperature to bake the tissue on to the glass and any evaporated water trapped between the specimen and the glass slide to enhance the chances of not losing the tissue.
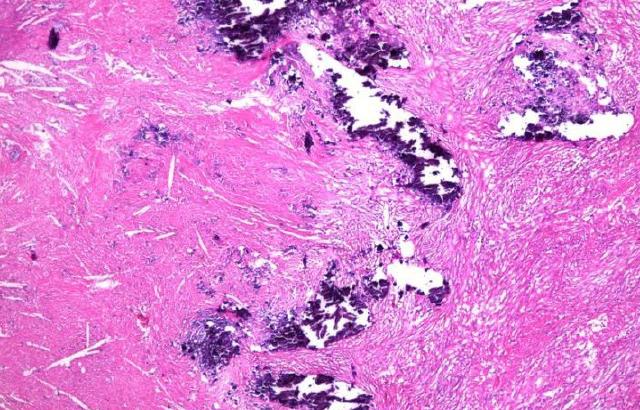
Since there's a non-chemical bond on those non-coated slide, how would you protect the specimen? A nightmare for a histology lab is when a tissue is placed on a piece of glass and the tissue just falls off or partially lifts off, and you have to go back and redo the experiment. This happens to specific tissue types, as pictured below. Activating tissue on the stainer could result in losing tissue when non-coated slides are used because there are no electrostatic forces or chemical bonds involved.
Application-related tissue loss due to tissue types
These are examples of application-related tissue loss. With immunohistochemistry, the tissue is exposed to high temperatures and repeated washes with different buffers, pH, or antigen retrieval techniques that require high temperatures, enzymes, or chemicals to dislodge tissue from the glass slides. With in situ hybridization high temperatures and the prolonged stringency washes are used, so it could be dangerous to the tissue if you don’t use coated slides. With frozen sectioning, non-fixed tissue is likely to lift off and tissue cut on a cryostat is thicker than your standard tissue thickness, 6 or 7 or even 10 microns, so also likely to lift off from the slide. Lastly, certain special stains involving heat or abrasive chemicals could result in tissue loss. You might want to consider something better than non-coated slides, something that will help you protect that precious specimen.
The tissue types that are specifically prone to detaching from the slide are skin, bone, cartilage tissue, dental tissue, retina or biopsy of the brain, which is basically fatty tissue. Samples cut at different-than-usual thicknesses, nerve cells or samples with more than one tissue type often float away from the slide.
What more can we do to protect the tissue than just heating the sample in an oven overnight? The most common way is to coat the glass slide with an adhesive. Adhesives generally bind with hydroxyl groups which are on the glass surface. They expose the amino groups, which are positively charged. Most animal tissues have a negative charge, so they seek the positive charges on the glass slide, creating a covalent bond that is so much stronger.
Nomenclature
To clarify the nomenclature, which is often confusing to people, there are positively-charged slides, named because of the positive charge; coated slides, named because the glass is coated; adhesive slides, named because the glass is coated with chemicals; hydrophobic slides, which were used more in the past, named because they are coated and repel liquid; polylysine slides, named because they are coated with polymerized amino acid, lysine; silane slides, named because they are coated with polymer/APES, which in my mind are a little better than amino acids or proteins; and sticky slides, which are referring to the positive and negative attraction on the slides.
Common Additives
When looking at additives used on the slides, amino groups/NH2 groups stick out. These are proteins used to coat the slide to attract negatively-charged tissue. Common additives include albumin found in milk or blood serum, gelatin from collagen, Poly-L-lysine, and cordia myxa, which is a flowering tree from Asia. Some use Elmer’s Glue as an additive, which used to be made from casein, a protein with NH2 groups.
Hematoxylin Background
There is a trade-off with using protein-based additives such as Elmer’s Glue or gelatin because they have NH2 groups, but also hematoxylin. The hematoxylin non-specifically binds to the negatively-charged carboxyl groups in large proteins, creating a background on the slide. Unwanted background on the slide can be cleaned with a variety of different acids. Acids will break the bonds between hematoxylin and the large proteins or even something on the glass such as protein that coats the slide with a blue tint. Strong acids would be used for a few seconds, while weak acids require extended time.
Here are pictorial representations of a slide with a background and a slide where the background has been removed.
Silanes
Let’s talk about silane. What is silane? Silane is a chemical formula of SiH4, one silica atom and four hydrogen atoms that has a nauseous smell. It is similar to acidic acid. Silane is used to dissolve in water or alcohol. It's very low percentage. People now use 3-aminopropyltriethoxysilane (silane or APES) to coat the slide. Aminosilane is a simple formula with the NH2 group, but you’ll notice there are no carboxyl groups. Therefore, this compound will not attract hematoxylin and will not create a background on the slide, making cleaner staining. During the manufacturing process, the silane is polymerized (hydrolyzing step) so it becomes one big polymer on the surface on the glass slide. Some also call silane a coupling agent because it couples in between the glass slide and the tissue. This graphic shows an aminosilane and the substrate surface or surface of the glass slide. With a certain amount of time and a certain temperature, the hydroxyl group from the substrate attaches to the silica through the oxygen bond and releases the water.
Factors Determining Slide Characteristics
Why aren’t all coated slides identical? Why do slides from different companies differ from one another? Everybody does it differently. The thickness of the silane layer matters; it is the first difference. This graphic shows a bunch of aminosilanes together polymerized with the glass slide at the bottom. How many layers would you position on the glass slide? If you’re not careful, you can have 20, 30, 40, 60 layers. There are scientists that actually count those layers. From published research, we know it needs to be no more than three to eight molecular layers. Too many layers will cause instability and you could lose the tissue. We don’t know how many layers there are on slides from different companies.
The extent of the covered surface is the second difference. From this picture, you can see the glass is green and the aminosilanes on top of it create a bond. However, this aminosilane did not react to the hydroxyl group of the glass and became an unreacted group. This is detrimental and if it happens a lot, it means the merging of the chemical was not ideal and it will not be as good as a silane reacting with the glass.
Distribution of the silane of the surface glass is another difference. Was the silane sprayed, was it dipped or was it brush painted onto the slide? The method might create different amounts of layers on the glass slide. Spraying allows for the most control of the amount of silane on the slide, and so is the best method.
Silane concentration makes a difference. Silane is never used in 100% concentration. I have seen 0.25% all the way up to 5%. Whether the higher or lower concentration is better will depend on who you talk to. Different concentrations have been tried in different manufacturing processes. In addition, silane dissolves equally well in water or alcohol, but when baking the slide, alcohol seems to evaporate more completely, allowing for better control.
During the manufacturing process, the number of washes of the slide when the silane is positioned makes a difference. After washing, the drying settings was also important, so people will play with different temperatures to see how the end product behaves.
All the above factors determine different slide characteristics and therefore, none of the slides are identical. In addition, there are about 3,000 different aminosilanes, so the question is which one you are using.
Coated Slides: Differences
Once you coat the slides, you create something called a hydrophobic environment. The picture here shows the glass slide in black and the specimen, perhaps a drop of blood or antibody, in red. That drop will be repelled because of the hydrophobic forces of the chemical, creating a dome on the surface. Depending on the type of glass, how much silane is on the glass, how you baked it, etc. you might have a different slide. The slides on the bottom are hydrophobic, meaning they’re both coated. The contact angle or wettability will depend on which silane or how much silane is used. Therefore, hydrophobic slides from the same company could still have different properties. We in histology labs know how to use these differences to our advantage; for example, immunohistochemistry or special stains.
To demonstrate the hydrophobicity the picture on the left shows water droplets or morning dew beading nicely on a leaf called a lotus effect, 135 degrees contact angle. The leaf on the right has also a hydrophobic surface, but the wettability is different.
On the other hand, uncoated slides display hydrophilic properties, meaning water-loving. The liquid on the surface will not bead up and create a contact angle of approximately 15 degrees. These are the slides that do not have any charges and there are no forces repelling the liquid.
This picture shows examples of different wettability. This is a 50 microliter drop of water on the left on a super hydrophobic slide and poor wettability. The water is repelled strongly, so it sits really high, maybe 5, 6 mm. The slide in the middle behaves differently, still being hydrophobic, but with a hydrophilic twist. The water occupies a bigger area and the contact angle is less, at about 40. The slide on the right is uncoated. There are no forces, so the water just spreads out and the contact angle is small.
Summary
In summary, not all glass is created equal. There is premium glass, the borosilicate glass, versus soda lime glass. Soda lime glass is not bad, it’s just that borosilicate glass has less impurity and better optic properties and thermal properties. Glass is inert unless the surface is modified. One atom of silicate with four atoms of hydrogen are not very friendly unless you force it by putting it in the oven and adding some chemicals that bind to a hydroxyl group.
Why do we lose precious specimens? Because we’re not careful or we don’t think we need to protect the tissue when we use immunohistochemistry, in situ hybridization, special stains. These are the techniques. Tissue like bone, ossified tissue, or retina needs to be protected; otherwise, it will be lost. We can use proteins or aminosilanes.
Proteins are very good, except there is a trade-off. Proteins will cause a background on the slide, which some people hate and others don’t mind, but you need to know where it comes from. The hematoxylin background comes from the hematoxylin binding to carboxyl groups of large proteins. It can be removed a little bit, but not entirely. This is why aminosilanes are much better, because they do not have carboxyl groups and do not cause a background.
All glass slides have different properties. We don’t know how different companies dissolve their silane, what temperature they use, or how they coat the slides. They use different chemicals in the manufacturing process to make the slides different. Of course, there is the notion of hydrophobicity that is created once the chemical is positioned on the surface of the glass slide and hydrophilicity of uncoated glass slides.
Q&A
Q: Would adding Tween-20 affect or help in hydrophilicity and keep specimens on the slide?
A: Yes and no. Tween-20 is used widely in molecular labs to break the surface tension. Breaking the surface tension will help with hydrophilicity so with something super hydrophobic, maybe the liquid will spread wider. However, it is unclear if it will help the specimen on the slide.
Q: Is there any specific type of slide that is best for in situ hybridization?
A: Good question. I’ve been working on in situ for 10 years. It is a nightmare task because you are targeting RNA or DNA and of course the dreaded DNase and RNase and destroy the whole experiment that might last days like radioactive in situ experiments would last weeks and weeks once coated in a photographic emulsion. First, those slides better be DNase or RNase free. If you buy an inexpensive slide touched by people, you might lose the target before getting to the real experiment. Quality is very important. You can use the heat to get rid of the DNase and RNase, but if you use heat, you might lose the modified surface of the glass. You could use chloroform, which is a little better. Chloroform kills DNase and RNase on contact without modifying silane, so silane would be inert.
In terms of in situ hybridization, I would inquire about the slide that is most hydrophobic. In my mind those hydrophobic slides have the most grabbing power. In situ hybridization is a dreaded application with so many washes with high temperatures, so you need all the help you can get to protect the tissue and keep it on the glass slide, tissue that is most hydrophobic and DNase- and RNase-free glass.
Q: When you were talking about various acidic solutions, do you have any specific recommendations?
A: If you mean differentiating agents, different companies use different products. I am not in the position to name specific products. In general, if you use regressive stains like Harris hematoxylin, then you must use diluted hydrochloric acid I believe 10 to 11 percent. This would remove what’s called a Harris Lake pool because you’re overstaining and then removing. When using Harris, you need hydrochloric acid diluted. Anything else, if using progressive stains, you can get away with the very weak organic acid. The time would be between 30 seconds and 1 minute 30 seconds. The weak organic acids like maleic acid, tartaric acid, citric acid, or acidic acid diluted in either alcoholr water are more aggressive. It’s easier or maybe safer to dilute a weak organic acid in 70% alcohol.
Q: What kind of slide is ideal when working with frozen tissue sections?
A: Again, without naming the slides, the answer would be the same as with the in situ hybridization, the most powerful hydrophobic slides you can find on the market.
Q: What is the best slide coating for bone tissue?
A: The question would be whether it is decalcified or non-decalcified. In general, the questions are all the same. You can see people ask me which slide is recommended in general. You have to understand that any hydrophobic, any adhesive or any coated slide is good. The difference is whether it is stronger or weaker. It’s a trade-off. If depends on you’re doing the experiment by hand or in an instrument. If you are doing it by hand, 10 slides and decalcified tissue, then I’ll go with the same answer that I gave the person that asked me about in situ hybridization, the most grabbing slide.
If the question were about immunohistochemistry, then I would say you don’t need the grabbing power. You just need a good coated slide with more hydrophilic properties, meaning your antibodies are more homogenously spread across the slide.
For a decalcified bone tissue and a process done by hand, the answer is the slide with the most grabbing power or the most hydrophobic with the biggest contact angle.
Q: Do charged slides lose their charge over time?
A: Absolutely, yes. Usually manufacturers will assign an expiration date and the time would be one year. If you open a box, stuff oxidizes over time. Also, you have sales people travelling to different labs. What happens is they keep the slides in the trunks of their cars and on a warm summer day, the temperature in a trunk could get up to 50, 60, 70 degrees Celsius. Slides should be kept at room temperature. Even if the box is unopened, it could behave differently because the chemistry of the slides was changed by the temperature. Because of that, we assign the time of one year.
Q: Is there any labeling on the different slide packaging that can identify the hydrophobicity or the quality?
A: Unfortunately, I’m not aware of anyone doing it. The only way to learn is if you have a favorite manufacturer, reach out to their technical support or the sales rep that is working with you and ask that question. They may know exactly what slides are what. As far as I know, none of that information is placed on the box by anyone.
발표자 소개

Andrew Lisowski has almost 30 years of experience in histology and histotechnology. He attended veterinary school and earned his master’s degree in molecular biology. Andrew worked in histology, IHC and ISH labs, cell culture lab, performed in-vitro and in-vivo toxicology assays and was a member of a necropsy team. He worked for pharmaceutical companies, medical school and founded his own molecular and histology firms.
Related Content
라이카 바이오시스템즈 Knowledge Pathway 콘텐츠는 에서 이용할 수 있는 라이카 바이오시스템즈 웹사이트 이용 약관의 적용을 받습니다. 법적고지. 라이카 바이오시스템즈 웨비나, 교육 프레젠테이션 및 관련 자료는 특별 주제 관련 일반 정보를 제공하지만 의료, 규정 또는 법률 상담으로 제공되지 않으며 해석되어서는 안 됩니다. 관점과 의견은 발표자/저자의 개인 관점과 의견이며 라이카 바이오시스템즈, 그 직원 또는 대행사의 관점이나 의견을 나타내거나 반영하지 않습니다. 제3자 자원 또는 콘텐츠에 대한 액세스를 제공하는 콘텐츠에 포함된 모든 링크는 오직 편의를 위해 제공됩니다.
모든 제품 사용에 다양한 제품 및 장치의 제품 정보 가이드, 부속 문서 및 작동 설명서를 참조해야 합니다.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.