
Optimizing Microtomy, Case Assembly and IHC Testing Through Implementation of Automated Specimen Tracking
The Leica Biosystems Content and Evidence Team has partnered with a private, multi-specialty clinic to assess the impact of implementing automated specimen tracking into their laboratory. This laboratory is most interested in increasing efficiency and patient safety throughout their processes.
During the Optimization Assessment, the Content and Evidence Team observed current processes throughout the histologic process beginning with accessioning and following through to case assembly. As with many laboratories without automated specimen tracking, paper logs or pending reports are used to track samples and users. The staff at this facility currently use LIS generated pending reports to follow cassettes and slides through their processes. Technologists refer to the reports for necessary sample information and to initial the steps that they perform to those samples.
The Leica Biosystems Content and Evidence Team also observed the current processes involved in creating slides. Slide labels are created while grossing dictation is entered into the LIS . The labels are printed from the LIS for each case. The labels are then delivered to the histology area of the laboratory in large groups. The staff spend time placing the labels onto the selected color slides and sorting them by case type and number. When cassettes are ready for microtomy, the technologists search for the matching slides and create a batch. Because there is such a large possibility of matching errors, the laboratory performs a manual block check verification with the slides before sending them to a pathologist.
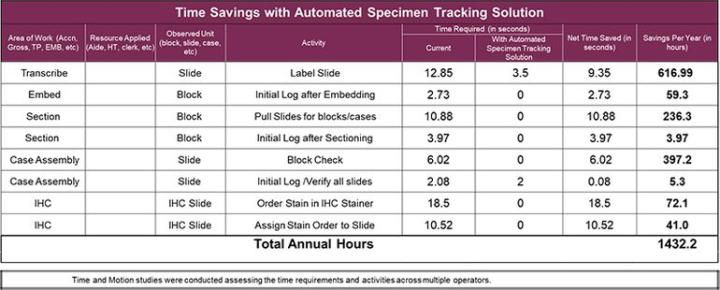
Through the implementation of an automated specimen tracking system, this laboratory could move towards eliminating the use of the LIS reports and change their labeling and slide creation process to a safer on-demand process. The resulting on-demand slide creation would be verified in the specimen tracking system to ensure that the tissue placed on the slide is from the correct cassette. This would help eliminate the chance of a mis-label during the labeling process in microtomy and reduce the safety risk to the patient. By eliminating these currently required manual steps, the average hands-on time would drop from 171.4 seconds to 147.2 seconds per block in microtomy.
Another area in which a specimen tracking system would increase efficiency is in immunohistochemistry. Since the specimen tracking system could be integrated with their IHC instrumentation, there would no longer be a need for the manual stain ordering and the assignment step. That integration could save more than 29 seconds per slide of hands-on time.
Since the specimen tracking system maintains the necessary information and tracks users throughout the process, the need to refer to or initial logs should be eliminated. The chart below shows specific steps and times savings that this laboratory could realize.
- Elimination of pending log used in 3 areas of the laboratory
- Save approximately 1432 hours/year by eliminating manual tasks
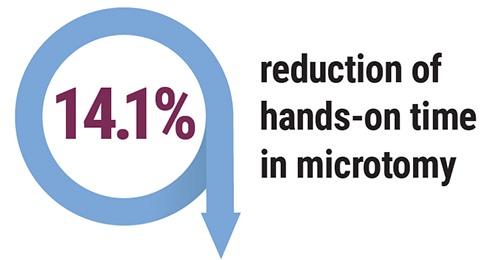
- Reduction of 14.1% hands-on time at microtomy
- Elimination of possible mis-label at grossing and microtomy
- Reduction of 29 seconds per slide in IHC
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
Leica Biosystems 콘텐츠는 Leica Biosystems 웹사이트 이용 약관의 적용을 받으며, 이용 약관은 다음에서 확인할 수 있습니다. 법적고지. 라이카 바이오시스템즈 웨비나, 교육 프레젠테이션 및 관련 자료는 특별 주제 관련 일반 정보를 제공하지만 의료, 규정 또는 법률 상담으로 제공되지 않으며 해석되어서는 안 됩니다. 관점과 의견은 발표자/저자의 개인 관점과 의견이며 라이카 바이오시스템즈, 그 직원 또는 대행사의 관점이나 의견을 나타내거나 반영하지 않습니다. 제3자 자원 또는 콘텐츠에 대한 액세스를 제공하는 콘텐츠에 포함된 모든 링크는 오직 편의를 위해 제공됩니다.
모든 제품 사용에 다양한 제품 및 장치의 제품 정보 가이드, 부속 문서 및 작동 설명서를 참조해야 합니다.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.