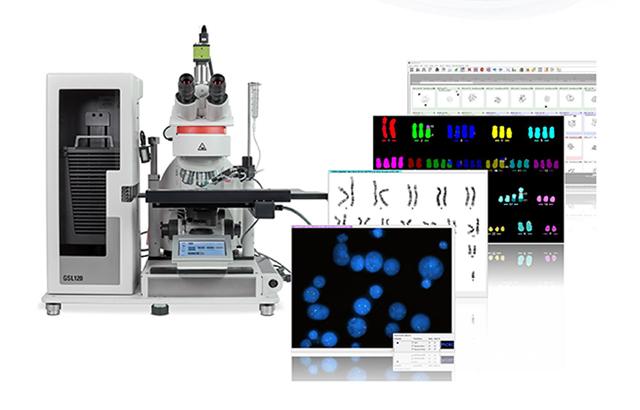
Leica Biosystems Launches IVDR Certified CytoVision DX – Complete Clinical Cytogenetics Platform
VISTA, California, September 30, 2025 – Leica Biosystems is proud to announce the launch of CytoVision DX, a next-generation image capture, analysis, and case management system purpose-built for clinical cytogenetics laboratories. Now CE-IVDR cleared and Windows 11 compatible, CytoVision DX sets a new standard in precision, automation, and connectivity for cytogenomics workflows.
Developed in collaboration with leading cytogenetics laboratories and with more than 30 years of proven performance, CytoVision DX delivers intuitive workflows to reduce hands-on time, enhance productivity, and streamline the cytogenetics process, from slide to report. Excellence in image quality combines with system flexibility, enabling high-resolution capture of brightfield or fluorescent samples for karyotyping, interphase FISH, tissue FISH, and more.
“We are delighted to introduce the IVDR-certified CytoVision DX, providing the flexibility, ease-of-use, and image quality that clinical cytogenetics demands,” said Naveen Chandra, Vice President and General Manager of Digital Pathology at Leica Biosystems. “The CytoVision platform has been a cornerstone for cytogenetics laboratories for over three decades, and this latest release delivers the regulatory, cybersecurity, and IT requirements for modern, innovative clinical practice.”
CytoVision DX delivers:
- Automated Intelligence, Human Expertise: Enable your technologists to focus on what matters most—analysis. CytoVision DX automates the time-consuming steps of identifying, locating, and capturing high-resolution interphase and metaphase cells, reducing hands-on processes and accelerating time to results.
- One Platform. Many Applications: From karyotyping and m-FISH to tissue FISH scanning, CytoVision DX supports mixed batch scanning in a single session with seamless integration.
- Image Clarity That Drives Confidence: Powered by the Leica DM6 B microscope and a 12 MP CMOS camera, CytoVision DX delivers exceptional image quality for chromosome banding and fine-detail review.
- Secure and Connected: Built for today’s cybersecurity standards with Windows 11 compatibility, user access controls, and remote review capabilities—ideal for multi-site collaboration.
- Scalable and Future-Proof: Hot-swapping trays, compact footprint, and interoperability with third-party IMS make CytoVision DX a flexible solution for labs of all sizes.
Click here for more information or to schedule a call with your local CytoVision DX expert.
For In Vitro Diagnostic Use
About Leica Biosystems
Leica Biosystems is a cancer diagnostics company and a global leader in anatomic and digital pathology solutions. The company offers a comprehensive product range for each step in the pathology process, from sample preparation and staining to imaging and reporting. As the only company to own the full workflow from biopsy to diagnosis, Leica Biosystems is uniquely positioned to break down the barriers between each step. The company’s mission of “Advancing Cancer Diagnostics, Improving Lives” is at the heart of its corporate culture. Leica Biosystems is proud to be a Danaher Corporation subsidiary.