
Reducing Batch Size to Improve Slide Turnaround Time
The Leica Biosystems Process and Solutions Optimization team has partnered with Queen Elizabeth Hospital to examine how to optimize their processes to reduce turn-around-time. QEH is most interested in having slides assigned to their Pathologists earlier in the day. The Process and Solutions Optimization team identified a few changes to help recognize and monitor their goals.
Following a workflow assessment, it was determined that the large batch sizes in use were causing delays and creating large wait times in several steps of the Histology processes. It was recommended that the laboratory reduce the batch sizes from embedding to case assembly in order to decrease the wait times and result in slides being completed earlier.
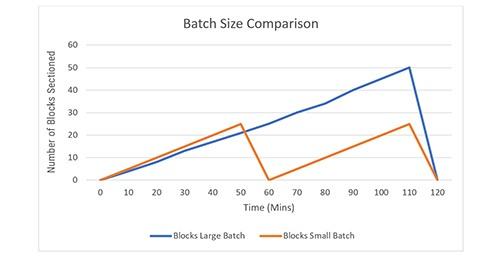
Figure 1 shows the comparison between large batches and small batches. Using a large batch takes more time to complete, whereas the smaller batch allows work to move to the next step in the process more quickly.
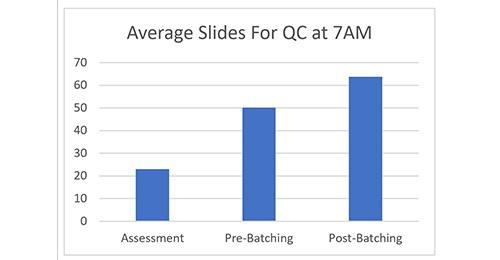
Figure 2 shows the average daily number of slides ready for QC by 7AM (embedding starts daily at 5AM). The Assessment data was collected during the on-site assessment in March 2018. The Pre-Batching data was collected by QEH staff in April 2018. The Post-Batching data was collected by QEH staff after the switch to smaller batch sizes in May 2018. That resulted in a 177% improvement in slides ready for QC compared to the assessment.

During the initial assessment in March 2018, the Pathologists had complained that on most days they did not receive any slides until after 10AM. In the initial month after the switch in batch sizes, slides were ready for the Pathologist on average by 8:09AM.
Projections and Realized Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.
El contenido de Leica Biosystems Knowledge Pathway está sujeto a las condiciones de uso del sitio web de Leica Biosystems, disponibles en: Aviso legal.. El contenido, incluidos los webinars o seminarios web, los recursos de formación y los materiales relacionados, está destinado a proporcionar información general sobre temas concretos de interés para los profesionales de la salud y no está destinado a ser, ni debe interpretarse como asesoramiento médico, normativo o jurídico. Los puntos de vista y opiniones expresados en cualquier contenido de terceros reflejan los puntos de vista y opiniones personales de los ponentes/autores y no representan ni reflejan necesariamente los puntos de vista ni opiniones de Leica Biosystems, sus empleados o sus agentes. Cualquier enlace incluido en el contenido que proporcione acceso a recursos o contenido de terceros se proporciona únicamente por comodidad.
Para el uso de cualquier producto, debe consultarse la documentación correspondiente del producto, incluidas las guías de información, los prospectos y los manuales de funcionamiento.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.