
Digital Pathology in Toxicological Pathology Studies

Drug development is a timely and costly process, 1 with the average cost of bringing a single new drug to market costing $1.3 billion, 2 taking anywhere between 10-15 years to finalize complete all clinical trials. 3 Preclinical studies are the vital first steps in research and development and is estimated to cost $7M over 73 months. 4 Up to 35% of drugs are eliminated at phase I and II clinical trials due to toxicity. 5
Toxicological pathologists are vital to the drug discovery process to identify efficacious and toxic effects of new drugs by histopathological analysis of animal model tissues that determines the no adverse effect level that will be used for phase 1 clinical trials. 6
Peer review is commonplace for toxicology pathology to solidify and enhance scientific accuracy and quality of the findings. 7 These findings could determine whether the compound should be pursued or terminated based on safety and efficacy. 6 It is important to make such decisions early in the drug discovery process to improve the return on investment for pharmaceutical companies. 6
However, many toxicological pathologists are faced with an inefficient workflow that prolongs the time for peer review due to slide packing, delivery, annotation etc., as well as geography of collaborators. The diagram below depicts the conventional peer review process.

Digital pathology can help to eliminate this workflow challenge when implemented into the toxicology pathology workstream. Digital pathology is the process of scanning whole slides to produce a digital image of the tissue that can be sorted, annotated, and shared virtually on a computer or mobile device. Digital pathology produces images at the same quality and resolution as the conventional microscope. Below is how the manual workflow can be changed with digital pathology:

In toxicological studies, additional advantages of digital pathology are becoming more widely recognized. This includes, but is not limited to:
- Reduce duplication of annotations and reports. 1
- Compare multiple slides side by side for analysis. 1
- Use metadata or organize, select, and sort slides for review within designated software. 1,8
- Reduce cost of pathologist’s travel and slide shipping, as well as reducing risk of damage to fragile slides. 8
- Standardize pathology and training for consistency of interpretation. 1
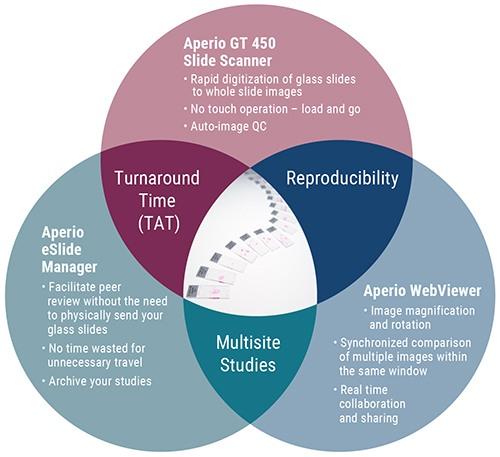
Leica Biosystems offers Aperio Digital Pathology solutions for whole slide scanning, web viewing and data management. This is highlighted in the diagram below:
About the presenter

Rhian is a Scientist from Swansea University in Medical and Healthcare Studies and was featured in several collaborative publications. Rhian’s research-based background focused on tissue-based pathology in Multiple Sclerosis, primarily using immunohistochemical analysis and in vitro molecular techniques. She spent a short period conducting routine PCR testing for COVID-19 at the end of 2020.
Referenzen
- Potts SJ. Digital pathology in drug discovery and development: multisite integration. Drug discovery today. 2009;14(19/20): 935-941.
- Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844–853. doi:10.1001/jama.2020.1166
- Cancer Research UK. How long a new drug takes to go through clinical trials. Cancer Research UK website. February 22, 2019. Accessed May 6, 2021. www.cancerresearchuk.org/find-a-clinical-trial/how-clinical-trials-are-planned-and-organised/how-long-it-takes-for-a-new-drug-to-go-through-clinical-trials
- Vieira M. Research synthesis: Costs of Pharmaceutical R&D. The Graduate Institute of Geneva website. January, 2020. Accessed May 6, 2021. da7af2c8-d9b0-47a3-a3f6-89c3c3bfa02c.filesusr.com/ugd/356854_e9d75e29c0264bf9b38118fc5f0aeab6.pdf DocuSign Envelope ID: FCD13E60-AFFE-41BF-856D-D9A6D04254EB
- Donowitz M, Turner JR, Verkman AS, Zacho NC. Current and potential future applications of human stem cell models in drug development. J Clin Invest. 2020;130(7):3342-3344.
- van Tongeren S, Fagerland JA, Conner MW, et al. The Role of the Toxicologic Pathologist in the Biopharmaceutical Industry. International Journal of Toxicology. 2011;30(5):568-582. doi:10.1177/1091581811413304
- Morton D, Sellers RS, Barale-Thomas E, Bolon B, George C, Hardisty JF, Irizarry A, McKay JS, Odin M, Teranishi M. Recommendations for pathology peer review. Toxicol Pathol. 2010 Dec;38(7):1118-27. doi: 10.1177/0192623310383991. Epub 2010 Oct 5. PMID: 20924082.
- Hamilton PW, Bankhead P, Wang Y, et al. Digital pathology and image analysis in tissue biomarker research. Methods. 2014;70(1):59-73. doi.org/10.1016/j.ymeth.2014.06.015
Related Content
Die Inhalte des Knowledge Pathway von Leica Biosystems unterliegen den Nutzungsbedingungen der Website von Leica Biosystems, die hier eingesehen werden können: Rechtlicher Hinweis. Der Inhalt, einschließlich der Webinare, Schulungspräsentationen und ähnlicher Materialien, soll allgemeine Informationen zu bestimmten Themen liefern, die für medizinische Fachkräfte von Interesse sind. Er soll explizit nicht der medizinischen, behördlichen oder rechtlichen Beratung dienen und kann diese auch nicht ersetzen. Die Ansichten und Meinungen, die in Inhalten Dritter zum Ausdruck gebracht werden, spiegeln die persönlichen Auffassungen der Sprecher/Autoren wider und decken sich nicht notwendigerweise mit denen von Leica Biosystems, seinen Mitarbeitern oder Vertretern. Jegliche in den Inhalten enthaltene Links, die auf Quellen oder Inhalte Dritter verweisen, werden lediglich aus Gründen Ihrer Annehmlichkeit zur Verfügung gestellt.
Vor dem Gebrauch sollten die Produktinformationen, Beilagen und Bedienungsanleitungen der jeweiligen Medikamente und Geräte konsultiert werden.
Copyright © 2024 Leica Biosystems division of Leica Microsystems, Inc. and its Leica Biosystems affiliates. All rights reserved. LEICA and the Leica Logo are registered trademarks of Leica Microsystems IR GmbH.

