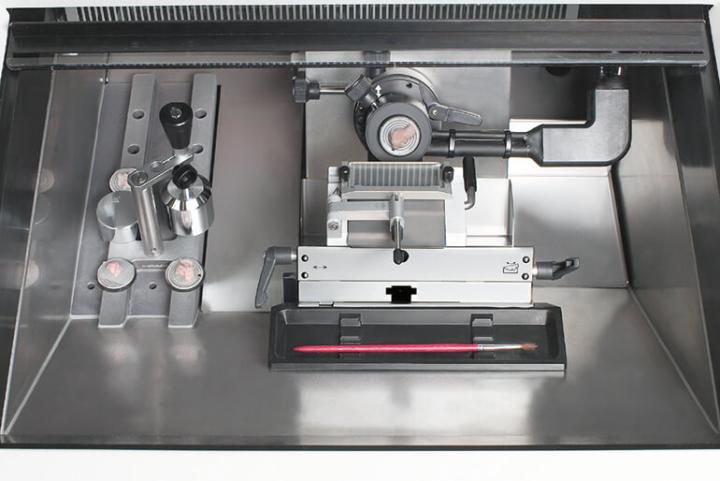
Precision Sectioning
When working with large samples, accurate specimen orientation and feed are crucial. The precise specimen orientation system with zero position provides for x/y adjustment of up to 8°. The specimen feed system with 25mm horizontal feed allows for reproducible thin sections.